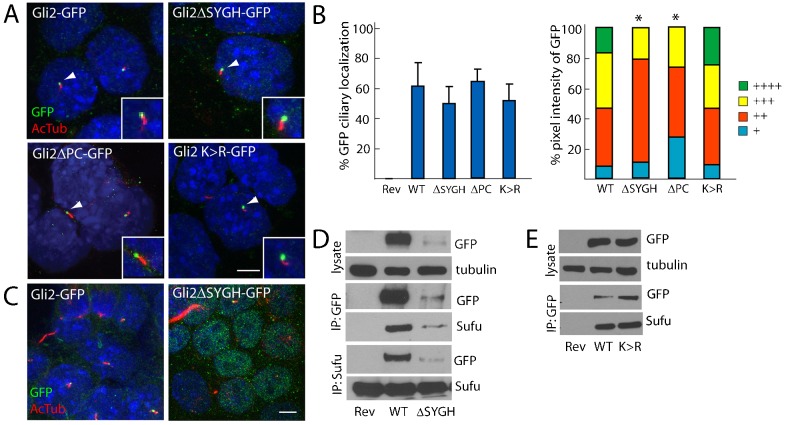
Fig. 3.
The interaction with Sufu, phosphorylation and sumoylation are not required for the ciliary localization of Gli2. (A) GFP-tagged Gli2, Gli2ΔSYGH, Gli2ΔPC and Gli2 K>R ESCs were stained for cilia (AcTub, red) and GFP (inset of individual cilium, white arrowhead). Scale bar: 5 µm. (B) The ciliary localization prevalence and pixel intensity of Gli2ΔSYGH–GFP (ΔSYGH), Gli2ΔPC–GFP (ΔPC) and Gli2 K>R–GFP (K >R) were compared to those of Gli2–GFP (WT). + denotes lowest pixel intensity, whereas ++++ denotes highest pixel intensity. *P<0.05 (Chi-squared test, as compared to WT). (C) Gli2ΔSYGH–GFP exhibited increased nuclear localization compared to Gli2–GFP, similar to Gli2-78–GFP, Gli2ΔRep–GFP and Gli2ΔAct–GFP. (D) Gli2Rev/+ (Rev), Gli2Gli2–GFP/+ (WT) and Gli2Gli2ΔSYGH–GFP/+ (ΔSYGH) ESC lysates were blotted for GFP and tubulin. The lysate was immunoprecipitated with anti-GFP or anti-Sufu, and blotted for GFP and Sufu. Gli2ΔSYGH–GFP protein levels were less than those of Gli2–GFP. Gli2ΔSYGH–GFP interacts with Sufu. (E) Gli2Rev/+ (Rev), Gli2Gli2–GFP/+ (WT) and Gli2Gli2K>R–GFP/+ (K>R) ESC lysates were probed for GFP and tubulin, or were immunoprecipitated with anti-GFP and immunoblotted for GFP and Sufu. Gli2 K>R–GFP protein levels are equivalent to Gli2–GFP and both can interact with Sufu.