Abstract
Within the tumor microenvironment, IL-6 signaling is generally considered a malevolent player, assuming a dark visage that promotes tumor progression. Chronic IL-6 signaling is linked to tumorigenesis in numerous mouse models as well as in human disease. IL-6 acts intrinsically on tumor cells through numerous downstream mediators to support cancer cell proliferation, survival, and metastatic dissemination. Moreover, IL-6 can act extrinsically on other cells within the complex tumor microenvironment to sustain a pro-tumor milieu by supporting angiogenesis and tumor evasion of immune surveillance. A lesser known role for IL-6 signaling has recently emerged in which it plays a beneficial role, presenting a fairer face that opposes tumor growth by mobilizing anti-tumor T cell immune responses to attain tumor control. Accumulating evidence establishes IL-6 as a key player in the activation, proliferation and survival of lymphocytes during active immune responses. IL-6 signaling can also resculpt the T cell immune response, shifting it from a suppressive to a responsive state that can effectively act against tumors. Finally, IL-6 plays an indispensable role in boosting T cell trafficking to lymph nodes and to tumor sites, where they have the opportunity to become activated and execute their cytotoxic effector functions, respectively. Here, we discuss the dual faces of IL-6 signaling in the tumor microenvironment; the dark face that drives malignancy, and the fairer aspect that promotes anti-tumor adaptive immunity.
1. Overview
Cancer is a disease of unrestrained growth where the normal mechanisms that regulate cellular expansion and division have been overridden. Carcinogenesis is generally thought to require disruption of two distinct pathways; removal of the checkpoints that limit cell division, and activation of signals to propel rapid growth [1]. However, there is growing evidence that the mechanisms underlying inflammation may help drive tumor formation, growth, and metastasis. Often described as wounds that do not heal, tumors bear many pathologic similarities to inflamed tissues [2–6]. Although a link between inflammation and cancer has been suggested for over a hundred years, the specific mechanisms linking inflammation to tumorigenesis have only recently begun to be elucidated [1,3–6].
The relationship between the immune system and cancer has long been a confounding issue. The immune system has strong potential to mediate anti-tumor activity and has been manipulated to treat cancer for over 100 years. Paul Erlich, one of the founders of the scientific discipline of immunology, devoutly believed that the immune system could be used as a ‘magic bullet’ to destroy cancer targets and began to test this hypothesis in mouse models of cancer in the early 1900s [7]. Because of its inherent target specificity, adaptability, and potential for lasting protection, the immune response remains a compelling target to treat cancer. Paradoxically, however, the picture that has emerged from analysis of ongoing immune and inflammatory processes within tumors indicates that inflammation can provide tumor-promoting signals and contribute to poorer outcomes [1,3–6]. Specific subsets of immune cells have been identified as key drivers of neoplastic progression, such as tumor-associated macrophages (TAM), neutrophils, myeloid-derived suppressor cells (MDSC), and CD4+ regulatory T cells (Treg) [1,4,6,8–10]. Many of these immune cells act through the secretion of cytokines including tumor necrosis factor (TNF), transforming growth factor-β (TGF-β), interleukin-1 (IL-1) and interleukin-6 (IL-6) [3–6,9–12]. Additionally, in some cancers (e.g., colon, renal cell, lung, and breast) secretion of these same cytokines by tumor cells helps drive and sustain pro-tumorigenic inflammatory loops [13–15]. Regardless of their cellular source, these immune mediators can create a favorable environment to support tumorigenesis by driving tumor cell proliferation, protecting tumor cells from cell death, and promoting angiogenesis and metastasis.
Of the proinflammatory cytokines, recent evidence suggests that IL-6 is a central player linking chronic inflammation to cancer by driving tumor initiation and subsequent growth and metastasis [4–6,12,16,17]. Further discussion on the pro-tumorigenic activities of IL-6 can be found in additional articles in the current issue of Seminars in Immunology. This review will focus on two opposing faces of IL-6 signaling in the tumor microenvironment, namely IL-6 as a critical driver of cancer formation as well as contributions of this pleiotropic cytokine to anti-tumor immunity by mobilizing T cell responses (Figure 1).
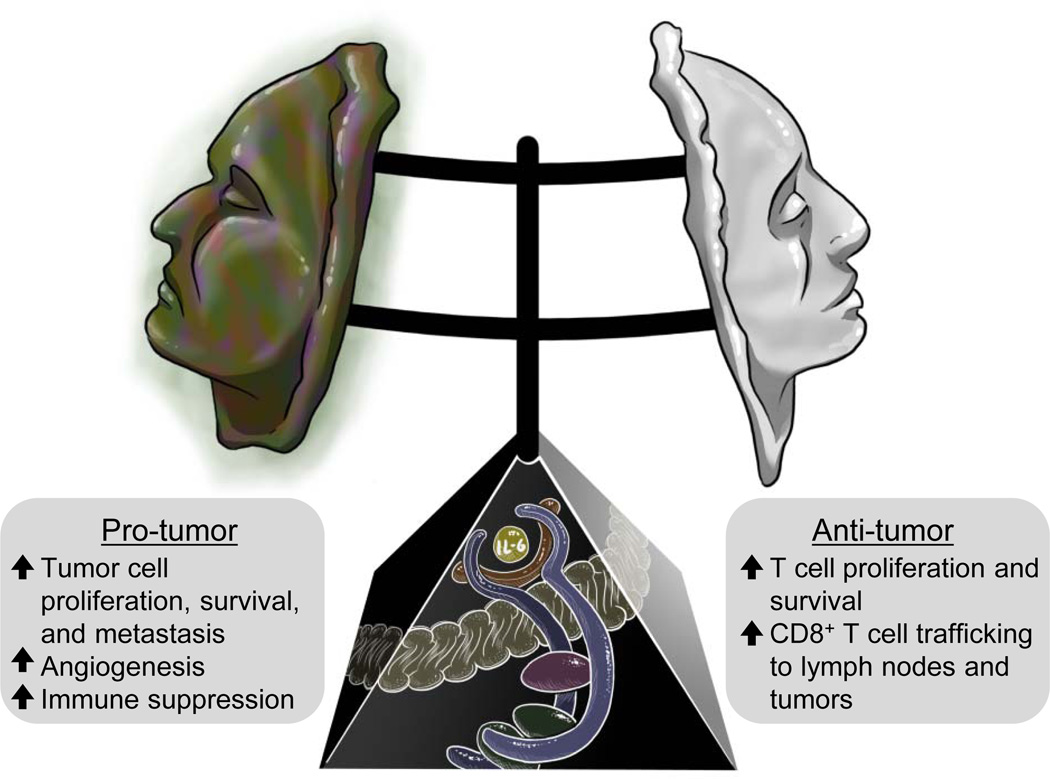
Figure 1. The dual faces of IL-6 trans-signaling in the tumor microenvironment.
IL-6 trans-signaling (shown in the pedestal) requires binding of IL-6 (yellow) to a soluble form of the IL-6 receptor α subunit (brown). This complex can then engage the membrane-anchored signal transducing receptor subunit gp130 (blue), leading to activation of downstream signaling molecules, e.g., JAK (purple) and STAT3/1 (green). Activation of IL-6 trans-signaling can take on a dark face in the tumor microenvironment supporting numerous pro-tumor activities (left). However, recent evidence has revealed that there is also a fairer face to IL-6 that promotes anti-tumor adaptive immunity (right).
2. Setting the stage: understanding the complicated path of IL-6 signaling
IL-6 signaling plays a complex role in inflammation. Although IL-6 has been described as anti-inflammatory in some settings, it also plays essential roles in promoting inflammation and immunity [12,16,18–20]. Originally identified as a B cell growth factor, IL-6 has been shown to provide important survival and proliferative signals to many leukocyte populations and orchestrates the development of the immune response. The myriad biological effects of IL-6 are accomplished through a tightly regulated signaling cascade [12,16,18–20]. Classical IL-6 signaling is initiated through the binding of IL-6 to the membrane-spanning, non-signaling IL-6 receptor α (IL-6Rα) subunit. The IL-6/IL-6Rα complex can then bind in cis to the signal transducing subunit glycoprotein 130 (gp130). One explanation for tight regulation of IL-6 signaling is that while gp130 is expressed ubiquitously on most cell types, expression of the IL-6Rα subunit is restricted mainly to hepatocytes and some leukocytes, thus effectively limiting the types of cells that can respond to IL-6 [12,16,18–20]. However, an additional mode of activation is available to IL-6 beyond classical signaling which involves a trans-signaling mechanism depending on a soluble form of the IL-6Rα (sIL-6Rα) released from cells, commonly by proteolytic cleavage, but potentially through alternate splicing of the IL-6Rα transcript.[12,16,18–20]. SIL-6Rα binds IL-6 directly in solution, allowing the formation of an IL-6/sIL-6Rα complex which binds in trans to activate membrane-anchored gp130 throughout the body. Trans-signaling, therefore expands the pool of IL-6-responsive cells to all gp130-expressing cells, regardless of their intrinsic expression of membrane-associated IL-6Rα [12,16,18–20]. Powerful experimental tools have been developed in order to definitively distinguish IL-6 functions that occur as a consequence of trans-signaling versus classical signaling [12,20]. In this regard, cellular responses have been examined using a recombinant fusion protein termed hyper-IL-6 (H-IL-6) in which IL-6 is covalently linked to sIL-6Rα, thus directly initiating trans-signaling responses [21]. On the other hand, soluble gp130 (sgp130) specifically antagonizes IL-6 trans-signaling without impeding classical signaling [12,17,19,22].
Binding of IL-6 to its receptors during both classical and trans-signaling converges on common pathways involving diverse intracellular signaling mediators [5,6,12,20]. IL-6/IL-6Rα ligation of gp130 induces auto- and trans-phosphorylation and activation of the Janus kinases (JAK) JAK1, JAK2, and Tyk2. JAK-phosphorylated tyrosine residues within the intracellular domain of gp130 serve as docking sites for downstream signaling molecules including STAT1, STAT3, SHP2, and PI3 kinase. These molecules function as transcription factors or enzymatic regulators of downstream signaling cascades that control transcriptional programs driving IL-6-dependent inflammatory responses that contribute to the multifaceted activities of IL-6 controlling tumor progression [5,6,12,20].
3. The dark face of IL-6 in the tumor microenvironment
IL-6 is most frequently cast as a malevolent character in the multi-act play encompassing cancer initiation and progression (Figure 2). Particularly damning are studies revealing that high serum concentration of IL-6 is a prognostic indicator of poor outcome in cancer patients with diverse histological tumor types including gastric, pancreatic, melanoma, breast, colorectal, myeloma, and lung cancer [23,24]. In murine models, IL-6 trans-signaling is linked to tumor development in inflammation-induced colorectal and pancreatic cancer [12,25–27]. Moreover, evidence that disruption of IL-6 trans-signaling delays growth in established murine tumors demonstrates that IL-6 activities are not limited to cancer initiation, but instead are also important during neoplastic progression [17,27]. The primary sources of IL-6 in the tumor microenvironment include tumor cells, per se, as well as TAM, MDSC, CD4+ T cells, and fibroblasts [13–15,25,27–32]. Recent studies in breast cancer, hepatocellular carcinoma, and lung cancer have highlighted how an epigenetic switch involving the miRNAs Lin28 and Let-7 allows tumor cells to produce IL-6 [31,32]. These changes support tumorigenesis as these tumor cells are able to survive using autocrine IL-6 production and do not depend on paracrine release of IL-6 by stromal cells. IL-6 and IL-6 trans-signaling can exert both tumor cell-intrinsic activities that directly affect cancer cells as well as tumor cell-extrinsic activities that indirectly support tumorigenesis by influencing stromal cells in the tumor microenvironment.
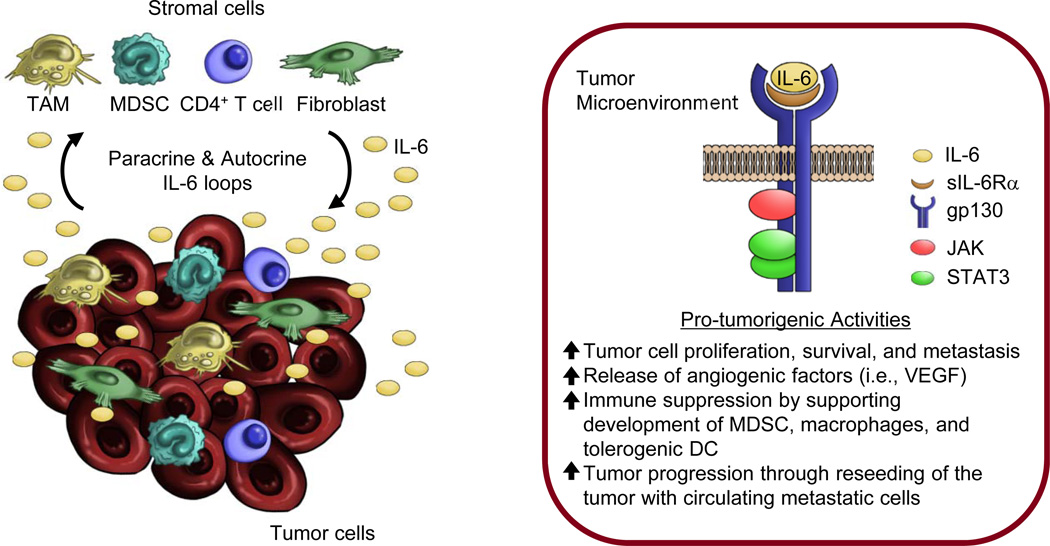
Figure 2. Sources and signaling of IL-6 in the tumor microenvironment.
Left panel, numerous inflammatory cells in the tumor microenvironment have been implicated in the production of IL-6 including tumor cells as well as local stromal cells (i.e., macrophage, myeloid-derived suppressor cells [MDSC], CD4+ T cells, and fibroblasts). Moreover, tumor cells themselves can produce IL-6 acting in an autocrine capacity. Right panel, IL-6 signals through a trans-signaling mechanism that involves binding of IL-6 to a soluble form of the IL-6 receptor α subunit (sIL-6R α). Engagement of gp130 by this multimeric complex leads to activation of downstream signaling molecules driving numerous tumor cell-intrinsic and extrinsic pro-tumorigenic activities in the tumor microenvironment.
Initiation of IL-6 signaling pathways in cancer cells activates mediators of cellular proliferation that are tightly controlled under homeostatic conditions. Dysregulation of STAT3 activation in cancer is an event so synonymous with cellular transformation that STAT3 is widely considered an oncogene [5,6,20,33–36]. Several studies have found that over 60% of inflammatory liver tumors have in-frame activating mutations of gp130, altering the IL-6Rα binding site or resulting in activation in the absence of ligand [35]. Activating mutations have also been found in STAT3, often in the SH2 domain which controls STAT3 dimerization, causing constitutive hyperactivation [36]. IL-6 trans-signaling-dependent activation of STAT3 can drive cancer progression through the transcription of target genes including the cell cycle regulator cyclin D1, the proto-oncogene c-myc, transcriptional regulators such as JunB, cFos and C/EBPβ and C/EBPδ, and metabolic regulators such as mTORC1 [5,20,33,34,37]. In the case of c-myc which is an important regulator of G1→S cell cycle progression, activated STAT3 binds directly to the c-myc promoter and helps drive c-myc transcription [34]. Interestingly, STAT3 binds at a site proximal to an essential E2F binding site, suggesting an alternative pathway regulating c-myc expression whereby STAT3 shields E2F from repressor complexes that normally limit c-myc activation by E2F. Activation of c-myc transcription further promotes proliferation by increasing cyclin D1 expression and repressing the cell cycle checkpoint protein p21 [34,38]. Beyond direct activity on cell cycle, IL-6 also acts via regulation of the mTORC1 metabolic sensor which has been linked to protein synthesis in highly active cancer cells. Excessive activation of mTORC1 following activation of gp130 in gastric cancer was shown to be required for inflammation-associated gastrointestinal tumorigenesis [37]. Moreover, blockade of mTORC1 activation suppressed tumor initiation and progression through a mechanism dependent on JAK and PI3K activity which can synergize with the activation of STAT3.
Acting through STAT3, IL-6 also supports tumor cell survival by inducing the expression of anti-apoptotic proteins including bcl2, bcl-XL and survivin [5,20,33,39]. Studies in human breast cancer cells found that expression of survivin is increased through direct STAT3 binding to the survivin promoter whereas STAT3 inhibition blocks survivin transcription and induces apoptosis of tumor cells [39]. Further, in myeloma cells, IL-6-dependent STAT3 signaling has been found to drive methylation and deactivation of the critical tumor suppressor gene p53, allowing cancer cells to bypass crucial checkpoints that regulate cell cycle progression and evade apoptotic signals resulting from DNA damage [40]. STAT3 signaling drives activation of DNA (cytosine-5)-methyltransferase 1 (DNMT-1), directly resulting in methylation and deactivation of the promoter region of p53 [40]. These studies also highlight the requirement for continued production of IL-6 in the tumor microenvironment, as persistent IL-6 signaling is required to maintain expression of DNMT-1 and the suppression of p53.
Activation of IL-6 signaling in tumor cells can additionally guide tumor growth by inducing factors that promote increased tumor invasiveness, metastasis and angiogenesis. The matrix metalloproteinases (MMP) are major mediators of these steps of tumor progression [41]. Specifically, increased expression of the proteinase MMP-2 is associated with STAT3 activation and more aggressive disease in melanoma models [42]. In this regard, constitutive activation of STAT3 in less aggressive tumors increases their metastatic potential. Conversely, loss of STAT3 signaling using a dominant negative STAT3 reduces MMP-2 expression and tumor metastasis [42]. IL-6 activation of STAT3 further drives angiogenesis by promoting expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor (bFGF) by tumor cells, supporting the rapid vascularization required for tumor growth and metastasis [43]. IL-6 has also been reported to promote tumor self-seeding by attracting the most aggressive metastatic cells out of circulation to primary tumor sites [44]. IL-6 supports tumor self-seeding by acting as an attractant for circulating metastatic tumor cells, helping guide them back into the primary tumor microenvironment where they contribute to tumor progression and metastasis.
In addition to the tumor intrinsic activities of IL-6, recent studies place IL-6 squarely at the epicenter controlling tumor growth by acting on the stromal constituents of the tumor microenvironment. For example, STAT3 activation initiated by IL-6 in tumor-associated endothelial cells, TAM, and MDSC induces their ability to express VEGF and bFGF in a feed-forward loop that positively regulates angiogenesis within tumor tissues [45]. Interestingly, recent evidence suggests that MDSC are not just targets of IL-6 trans-signaling activity, but they may also collaborate with tumor cells in a mutual activation loop that triggers both increased IL-6 production and the release of sIL-6Rα into the tumor microenvironment [30]. In mouse mammary tumors, high concentrations of IL-6 correlate with accumulation of MDSC which in addition to producing IL-6, also express the ADAM-17 metalloprotease that mediates IL-6Rα shedding from MDSC surface membranes, thereby enabling IL-6 trans-signaling to occur in the tumor microenvironment [12,30,46].
It is increasingly clear that the pro-tumorigenic activities ascribed to IL-6 extend far beyond direct effects on tumor growth by enacting suppression of the adaptive immune response. IL-6 reportedly interferes with the development of antigen-presenting cells such as dendritic cells (DC) that are necessary for priming cytotoxic T cells [47,48]. Activation of IL-6 signaling in myeloid progenitor cells can redirect their differentiation away from the DC lineage, causing them to preferentially polarize toward monocyte/macrophage cells, such as macrophages or MDSC, which are known suppressors of immune function [28,47]. Additionally, IL-6/STAT3 signaling blocks the maturation of differentiated DC, thus preventing T cell activation and inducing T cell anergy or death [48]. Collectively, the many unique mechanisms activated through IL-6 signaling that culminate in increased tumor growth, progression, metastasis, and evasion from immune protection have led to typecasting of IL-6 as a sinister actor in the tumor microenvironment.
4. The fairer face of IL-6 in adaptive immunity
While the predominant view of IL-6 in cancer is as a key driver of malignancy, there is a second face of IL-6 that has sparked interest due its beneficial role in promoting anti-tumor immunity [49,50]. Since its initial characterization as a mediator of humoral immunity, IL-6 has been shown to have broad effects on leukocyte survival, proliferation, differentiation, and recruitment [5,12,17,18,51–65]. These provocative observations suggest that IL-6 and IL-6 trans-signaling can potentially be harnessed to tilt the balance in the tumor microenvironment away from tumor cell growth and survival toward protective anti-tumor immunity (Figure 1).
The notion that the immune response is competent to act against tumors has waxed and waned in popularity over the past century. Analysis of large numbers of human tumor specimens led to the discovery that the immune contexture, defined by the frequency, type, activation, and distribution of immune cell infiltrates within tumor lesions, can be a better prognostic marker than histological staging [49,50]. The status of host adaptive immunity has surprisingly broad implications since CD8+ effector T cells have been shown to be necessary not only for anti-tumor immunity, but also for the efficacy of standard anti-cancer regimens including chemotherapy and radiation [66,67]. Although the good face of IL-6 in the immune contexture is not completely understood, there is building evidence that IL-6 trans-signaling is a key player in the mobilization of anti-tumor T cell responses [17,18,51,55–64]. In sections 4.1–4.3 we discuss evidence that IL-6 supports adaptive immunity by acting at 2 key sites: the lymph node, where it impacts lymphocyte, priming, subset programming and activation, and trafficking, as well as tumor locales where IL-6 promotes the delivery of effector T cells to the cancer microenvironment.
4.1. IL-6 regulates the priming environment of lymph nodes
Lymph nodes are the primary site for lymphocyte activation and development of adaptive immune responses to local insults such as infection and cancer. Antigens and antigen-loaded DC travel via afferent lymphatics that link inflamed tissues to draining lymph nodes where the tissue architecture and chemokine environment act cooperatively to facilitate rapid screening of DC by recirculating T cells. Naïve and central memory T cells enter nodes through specialized blood vessels termed high endothelial venules (HEV) and immediately interact with antigen-bearing DC whose strategic positioning adjacent to these unique vascular portals maximizes the opportunity for productive encounters with T cells expressing cognate T cell receptors. This is a highly efficient process such that under resting conditions, 500–5,000 T cells scan DC every hour [68]. If a given T cell does not encounter its cognate antigen within 8–12 hours after entry, it will exit the node and re-enter the blood circulation to continue recirculating [69]. However, if a T cell recognizes cognate antigen presented by DC, it remains in the lymph node in prolonged intimate contact with DC which provide activation and differentiation signals that shape the T cell immune response [68]. Lymph nodes also potentiate durable immunity to foreign pathogens or cancer cells by acting as a site of restimulation of memory T cells.
IL-6 plays a vital role in the development of T cell responses. In this regard, IL-6, which is largely produced by DC in the lymph node has been demonstrated to impact the activation, expansion, survival, and polarization of T cells during an immune response [70]. In various immunization settings, IL-6 signaling is required for optimal T cell priming, the induction of a productive IFN-γ response, protection of T cells from the suppressive activities of Treg, and the acquisition of the ability to provide help to B cells [71–73]. In addition to driving the development of CD8+ lymphocyte effector functions, IL-6 has also been described as promoting T cell proliferation following T cell receptor stimulation [5,52]. IL-6 further protects T cells from apoptotic death through the induction of the anti-apoptotic proteins bcl-2 and bcl-xL and the proto-oncogenes cFos and JunB [5,12].
A recently discovered immunostimulatory activity of IL-6 trans-signaling is the skewing of CD4+ T cells away from Treg toward the pro-inflammatory Th17 phenotype. In a murine model of aggressive melanoma, IL-6 was shown to be necessary for the alleviation of Treg-mediated immune suppression and for effective priming of CD8+ T cells in the tumor-draining lymph node [74]. Significantly, the differentiation of Th17 cells and Treg are interconnected; naïve CD4+ T cells stimulated in the presence of TGF-β express Foxp3 and become functional Treg. However, in the presence of both TGF-β and IL-6 trans-signaling, the Foxp3-dependent Treg developmental program is actively suppressed, and cells become Th17 [47, 48]. Considering the important role Treg play in immune suppression and tumor escape from immune surveillance, [44, 67] these results are highly suggestive that IL-6 trans-signaling can switch the host immune response from a suppressive state to an active state that supports anti-tumor immunity.
4.2. IL-6 trans-signaling promotes lymphocyte trafficking to lymph nodes
The ability to mount an adaptive immune response directed against pathogens or cancer ultimately depends on lymphocytes efficiently gaining access to the lymph node microenvironment. HEV are the major gateway for the entry of circulating naïve and central memory T lymphocytes or naïve B cells and are lined by cuboidal endothelial cells that express specific adhesion molecules and chemokines that support trafficking (Figure 3A) [69]. HEV-like vessels can also be induced at sites of chronic inflammation and have been identified in human solid tumors where they correlate with increased T lymphocyte infiltration and better prognosis [69]. Lymphocyte trafficking to lymph nodes via HEV occurs through a well-defined multistep cascade of adhesive and activation events (Figure 3B) [18,69]. This cascade is initiated by transient interactions between lymphocyte L-selectin (CD62L) and its HEV glycoprotein ligands, collectively known as peripheral lymph node addressin (PNAd). These short-term tethering and slow rolling interactions allow lymphocytes to survey the HEV surface for the chemokine CCL21. CCL21 signaling though the CCR7 chemokine receptor on lymphocytes increases the affinity of lymphocyte adhesion molecule (LFA-1), enabling it to mediate the firm arrest step via binding of its endothelial counter-receptors, ICAM-1 and ICAM-2, which is necessary for subsequent extravasation of lymphocytes and migration into the underlying tissue parenchyma [69]. Under resting conditions, lymphocyte migration through HEV is a highly effective process, with ~2×106 lymphocytes per hour entering and surveying all 22 lymph nodes in the mouse [75]. This high rate of homeostatic trafficking compensates for the relatively low frequency (~1–10 per 106 cells) of T cells that can recognize a given antigen. Remarkably, IL-6 trans-signaling was found to boost this already efficient system of lymphocyte trafficking to lymph nodes, acting both at the level of circulating lymphocytes and independently at the level of the HEV to increase immune surveillance [17,55–59,62,63].
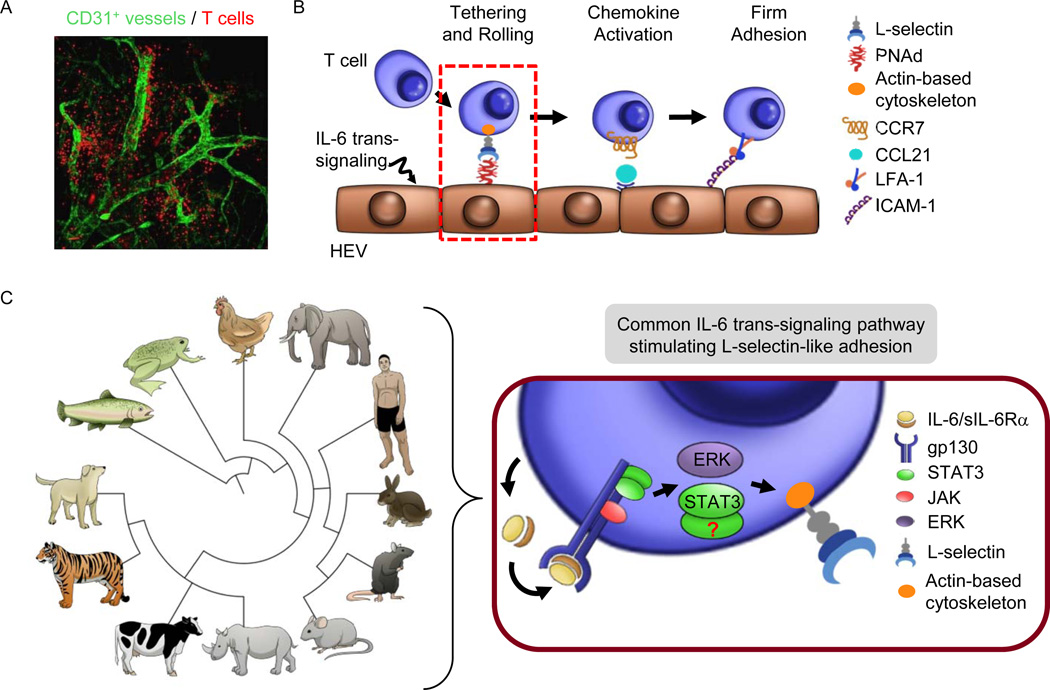
Figure 3. IL-6 trans-signaling acts on lymphocytes through an evolutionarily conserved mechanism to improve trafficking across high endothelial venules.
(A) Confocal whole-mount photomicrograph depicting highly efficient homing of naïve and central memory T cells (red) within lymph nodes in short-term homing assays performed as described [56,59,62]. Specialized vascular gateways known as high endothelial venules (HEV) constitute the majority of CD31+ (green) vessels shown in the photomicrograph. (B) IL-6 trans-signaling activated through H-IL-6 or thermal stress acts on lymphocytes to augment interactions between L-selectin and the actin-based cytoskeleton (yellow), which improves initial tethering and rolling interactions in HEV. (C) Left, IL-6 trans-signaling activates L-selectin-like adhesion under febrile conditions in lymphocytes of vertebrate species separated by >400 million years of evolution. The relationship between 4 major branches of vertebrate evolution (teleost, amphibian, birds, mammalian) are depicted based on NCBI taxonomy [86]. Right, IL-6 and sIL-6α produced by leukocytes can act through ERK1/2 signaling to activate increased L-selectin binding activity. It remains an open question if STAT3 plays a role in this process.
The impact of IL-6 trans-signaling on the trafficking potential of circulating lymphocytes emerged from studies investigating enhanced lymphocyte homing in response to febrile temperatures as a model of acute inflammation. Exposure of mice or human cancer patients to mild passive heating mimics the thermal element of a natural fever by raising the core temperature to a febrile range (~39.5°C) for periods up to 6 hours [51,76]. Systemic administration of febrile-range thermal stress results in the transient decrease in the number of circulating lymphocytes in both cancer patients and mice [59,60]. Lymphocyte counts recovered rapidly after cessation of thermal stress, supporting findings that heat treatment is not toxic to lymphocytes, but rather drives the redistribution of lymphoid cells from the blood into tissues [58,60].
Further studies revealed that exposure to febrile temperatures causes an ~2-fold increase in lymphocyte trafficking into lymph nodes in normal as well as in tumor-bearing mice [17,56,59,61,63]. The fact that this increase could be detected in homing experiments lasting for just 1 hour verified that the increased numbers of cells in lymphoid organs was not due to effects on their retention, survival, or local proliferation, thus isolating trafficking as the major thermally-responsive mechanism regulating lymphocyte accumulation in lymph nodes [57,61]. Heat-experienced lymphocytes exhibit enhanced L-selectin-mediated adhesion to HEV that was not associated with upregulation of L-selectin protein or with changes in its intrinsic ligand-binding activity. Rather, fever-range heating causes L-selectin to rapidly associate with the actin-based cytoskeleton, thereby stabilizing lymphocyte-HEV interactions [57,58,64]. Investigation of other lymphocyte trafficking proteins such as LFA-1 demonstrated that thermal stress has no effect on their expression or function [58].
The contributions of IL-6 trans-signaling to the regulation of L-selectin function under febrile inflammatory conditions began to come to light when it was observed that the conditioned medium from thermally-treated lymphocytes conferred all of the pro-trafficking activities observed in response to direct exposure of lymphocytes to febrile temperatures [57]. This result strongly suggested that soluble factors produced by leukocytes are key regulators of trafficking. Screening of a panel of recombinant pro-inflammatory cytokines identified several candidates (i.e., TNF, IL-1β, IL-6, and IFN-α) that are able to enhance L-selectin adhesion when added as recombinant proteins to lymphocyte cultures [55,57]. However neutralizing antibody studies demonstrated that IL-6 is the sole cytokine contained in conditioned medium of heat-treated cells that regulates L-selectin function [57]. Furthermore, treatment of lymphocytes with H-IL-6 was sufficient to boost L-selectin binding, whereas treatment with sgp130 prior to thermal stress abrogated these pro-adhesive effects, establishing that febrile temperatures initiate an endogenous IL-6 trans-signaling loop that regulates lymphocyte adhesion and trafficking [18,55,57]. No change was detected in the concentrations of either IL-6, sIL-6Rα, or sgp130 in conditioned medium from cells exposed to febrile temperatures or in circulation following elevation a core body temperatures to the febrile range of mice or advanced cancer patients to the febrile range ([17,56,57] and S.S.E., unpublished observations). These findings suggest that thermal stress may alter IL-6 bioactivity or bioavailability by an unidentified mechanism that does not involve new synthesis of key IL-6/IL-6R components. This is in contrast to observations that during acute inflammation driven by toll-like receptor-4 (TLR4) ligation, circulating IL-6 levels become elevated when mice are exposed to fever-range thermal stress [77,78]. Investigation of the intracellular signaling demonstrated that thermally-responsive IL-6 trans-signaling results in the activation of both STAT3 and ERK1/2 within lymphocytes. While functional blocking assays showed a clear requirement for ERK1/2 signaling, the role of STAT3 activation in the modulation of L-selectin function remains to be investigated [57].
The relationship between fever-range temperatures, IL-6 trans-signaling, and L-selectin adhesion to HEV was unexpectedly found to be evolutionarily conserved [55]. In this regard, exposure of leukocytes to febrile temperatures initiated IL-6 trans-signaling that promoted L-selectin-like adhesion in leukocytes from vertebrate species that shared a common ancestor >400 million years ago (i.e., mammals, birds, amphibians, and teleosts) (Figure 3C). Even more surprising, some of these animals lack HEV or specialized lymph nodes (e.g., amphibians, teleosts), suggesting a role for the prototypical L-selectin lymph node homing receptor in immune defense at extralymphoid sites of injury or infection. Evidence that IL-6 trans-signaling-dependent activation of L-selectin function occurs in isolated leukocytes exposed to heat in vitro indicates that both IL-6 and sIL-6Rα can be derived from leukocytes. This finding suggests that thermal stress regulates L-selectin function by initiating an endogenous loop of IL-6 trans-signaling in leukocytes, resulting in the activation of MAPK (i.e., ERK1/2) and STAT signaling pathways [55,57,58,64]. The remarkable evolutionary conservation strongly supports the concept that febrile activation of IL-6 trans-signaling arose as an important protective mechanism to generate effective immune responses.
Given the importance of IL-6 trans-signaling and febrile stress in regulating the adhesion of lymphocytes, studies were initiated to determine whether similar cytokine regulatory mechanisms control the intrinsic adhesive properties of HEV. To discriminate the effects of heat on the endothelium without complications from direct heat effects on circulating lymphocytes, an adoptive transfer approach was taken in which the fate of heat-inexperienced lymphocytes was assessed after their infusion into pre-heated mice [56,59,62,63]. These studies demonstrated that regardless of whether IL-6 is provided through an endogenous loop initiated by thermal stress or supplied as H-IL-6, it induces an ~2-fold increase in the ability of HEV to support lymphocyte trafficking into lymph nodes compared to control mice (Figure 4A) [56,59,62]. This IL-6 activity was observed in multiple mouse strains (C57BL/6, C3H, BALB/c), including SCID, indicating that enhanced HEV adhesion does not require the presence of mature lymphocytes in lymphoid organs [56]. Moreover, neutralization studies utilizing the sgp130 decoy receptor determined that thermal induction of lymphocyte trafficking is entirely dependent on IL-6 trans-signaling [56].
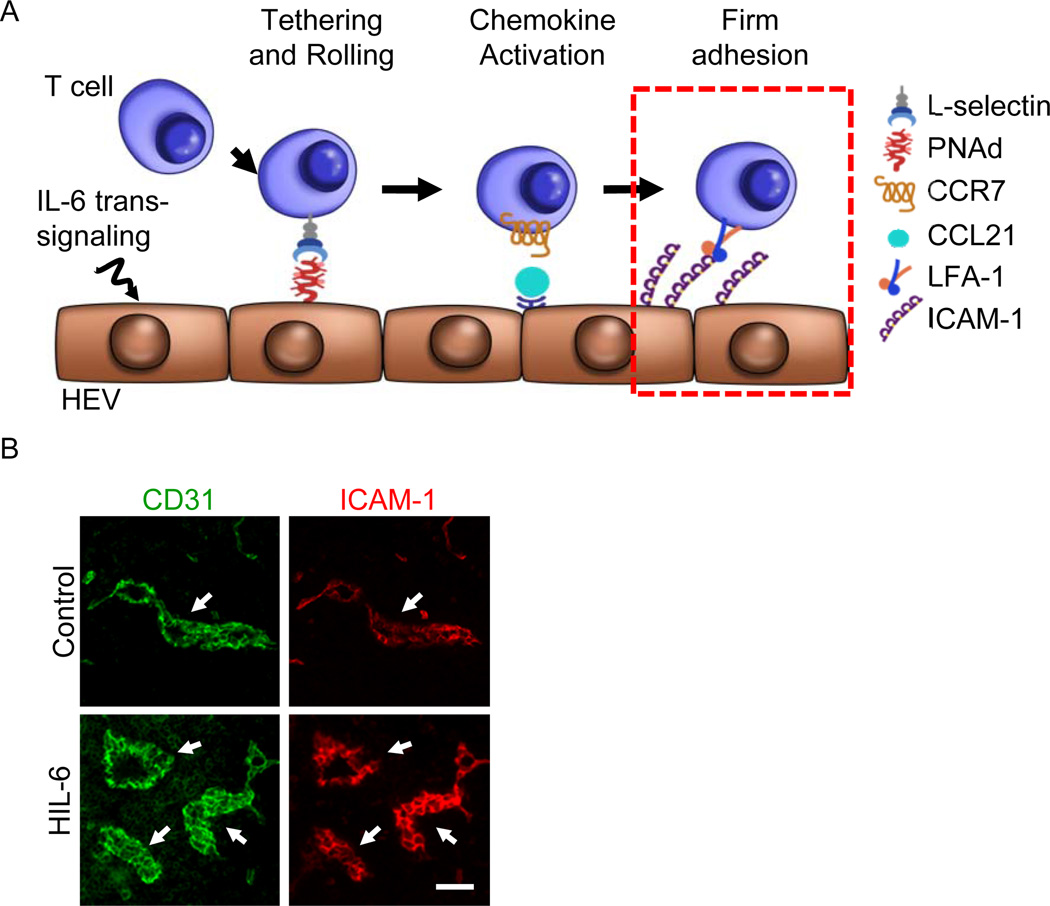
Figure 4. IL-6 trans-signaling acts on high endothelial venules (HEV) to augment ICAM-1 dependent trafficking of lymphocytes.
(A) IL-6 trans-signaling activated by H-IL-6 or thermal stress increases the intrinsic capacity of HEV to support firm adhesion of circulating lymphocytes. (B) Mice were injected with H-IL-6 (50 µg, i.v.), and 6 hours later, lymph nodes were immunostained for CD31 (green) and intravascular ICAM-1 (red) as described [17,56,62]. The position of HEV is demarked based on landmark CD31+ vessels (arrows). Size bar, 100 µm.
In order to dissect which steps of the trafficking cascade were altered by IL-6 trans-signaling, intravital microscopy imaging was used to visualize the interactions between circulating lymphocytes and HEV in real-time within surgically exposed lymph nodes of live mice [56]. Contrary to IL-6 trans-signaling responses in lymphocytes which stimulated rolling frequency, H-IL-6 and thermal treatment increased firm adhesion in HEV, thus excluding contributions of PNAd to enhanced HEV adhesion. This result pointed to 3 candidate trafficking molecules as potential targets of regulation by IL-6; the chemokine CCL21, ICAM1, and ICAM-2, all which are known contributors to the firm arrest step in HEV (Figure 4A). Semi-quantitative immunofluorescence microscopy revealed that the density of CCL21 and ICAM-1 are both increased on HEV in response to thermal stress while PNAd and ICAM-2 are unchanged [18,56,62]. Moreover, the effects of heat on ICAM-1 expression were paralleled by systemic administration of H-IL-6 (Figure 4B and [56]). In contrast, CCL21 was not induced by H-IL-6, suggesting that an unidentified IL-6-independent signaling pathway is also induced by thermal stress that regulates CCL21. An obligate role for IL-6 trans-signaling in increasing ICAM-1 expression and T cell trafficking following fever-range thermal stress was confirmed in loss-of-function studies where mice were pre-treated with sgp130 [56]. Thus, acute activation of IL-6 trans-signaling through direct application of H-IL-6 or through an endogenous loop initiated by febrile temperatures improves lymphocyte trafficking through lymph nodes by increasing ICAM-1 expression on HEV (Figure 4).
Taken together with the effects of trans-signaling on lymphocyte trafficking, these findings suggest a novel IL-6 trans-signaling mechanism that boosts immune surveillance and potentially supports anti-tumor immunity by influencing the delivery, activation, and polarization of T lymphocytes within lymphoid organs. IL-6 trans-signaling-dependent mechanisms that improve naïve and central memory T cell trafficking in lymph nodes could be particularly relevant in the context of the immunosuppressive environment of tumor-draining lymph nodes which are characterized by decreased rates of trafficking across HEV [79]. Moreover, the ability of IL-6 to act on lymph nodes throughout the body suggests that IL-6 trans-signaling has the potential to enhance immunity within lymph nodes draining both primary tumors and disparate sites of metastatic disease.
4.3. Mobilization of adaptive anti-tumor immunity to tumor tissues by IL-6 trans-signaling
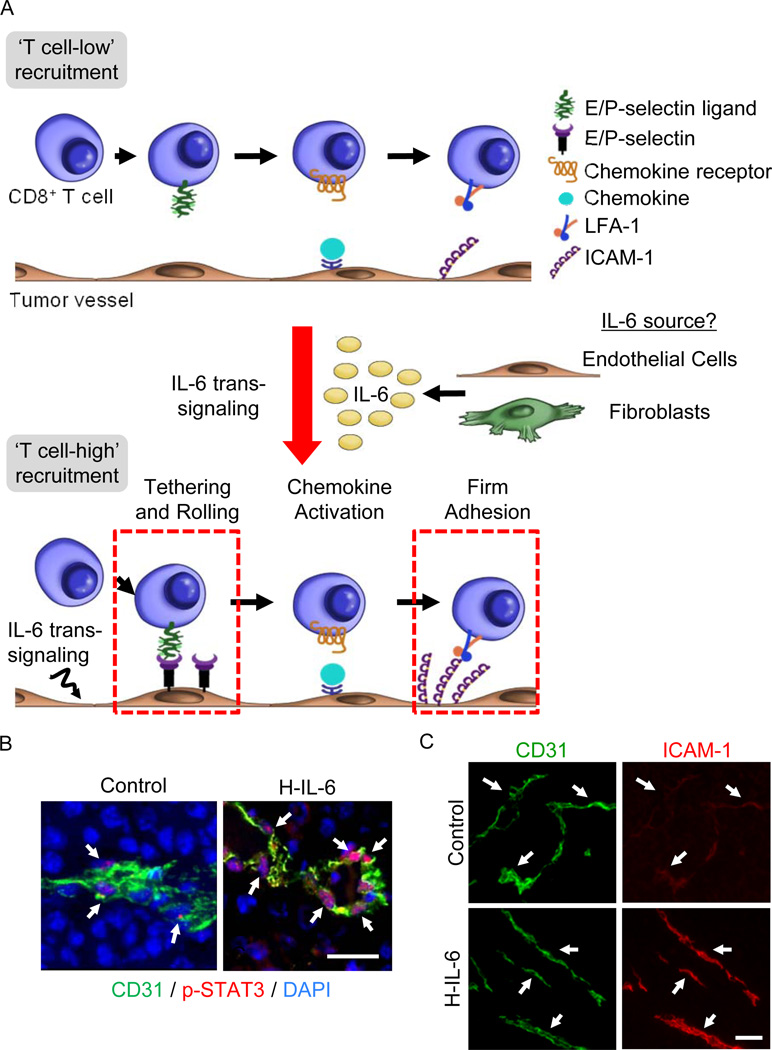
Given the prevailing view that IL-6 plays a central role in tumorigenesis and neoplastic progression, it would not readily be predicted that IL-6 signaling could also stimulate anti-tumor activities within the tumor microenvironment. However, evidence that IL-6 influences lymphocyte trafficking across HEV in lymphoid organs and at sites of inflammation raised the possibility that this cytokine might also contribute to CD8+ effector T cell trafficking within IL-6-rich tumor tissues. This question is particularly germane to the observed correlation between patient outcomes and intratumoral infiltration by CD8+ cytotoxic T cells since adaptive tumor immunity hinges, in part, on the rate of T cell trafficking at tumor vascular checkpoints. Specifically, circulating cytotoxic T cells must first navigate across tumor vascular barriers and enter the tumor parenchyma in order to initiate contact-dependent lysis of target cancer cells. Intravital microscopy studies revealed that despite ongoing IL-6 trans-signaling in tumors, tumor vessels were incapable of supporting a high rate of extravasation of circulating CD8+ T cells under homeostatic conditions (Figure 5A) [17]. These findings correlate with the peculiar nature of tumor vessels that have an irregular tortuous structure and express low levels of trafficking molecules such as ICAM-1 [17,51] Further, the failure of the tumor vasculature to support efficient CD8+ T cell trafficking could not be overcome using inflammatory stimuli such as the TLR4 agonist, lipopolysaccharide (LPS), or recombinant TNF which, in non-tumor settings, stimulate vascular endothelium to support leukocyte trafficking [17,80–82]. Insensitivity of tumor vessels to inflammatory signals is attributed to pro-angiogenic molecules, i.e., VEGF and bFGF, which condition the vascular endothelium to be unresponsive to inflammatory cues via epigenetic silencing of ICAM-1 expression [82]. Thus, while there is strong interest in developing therapeutic strategies to convert non-recruiting T cell low tumors to T cell high recruiting sites for effective anti-tumor immunity, the overall consensus is that targeting tumor microvessels for improved T cell homing remains a formidable challenge [83].
Figure 5. IL-6 trans-signaling improves CD8+ effector T cell trafficking across tumor vascular checkpoints.
(A) IL-6 trans-signaling activated via H-IL-6 or thermal therapy acts on tumor microvessels converting them from ‘T cell-low’ to ‘T cell-high’ recruitment sites by increasing both initial tethering and rolling interactions as well as firm adhesion between circulating CD8+ effector T cells and tumor vessels [17]. The cellular source of IL-6 required to support thermally induction of T cell trafficking has been identified as radiation-resistant nonhematopoietic cells, implicating fibroblasts or the vascular endothelium itself. (B) Mice inoculated with B16 melanoma tumors (s.c.) were treated with H-IL-6 (50 µg, i.v.). After 30 minutes, phosphorylated STAT3 (red) in DAPI-stained nuclei (blue) of CD31+ (green) tumor-associated endothelial cells was detected by immunostaining as described [17]. Size bar, 50 µm. Arrows denote CD31+ vessels. (C) Six hours following administration of H-IL-6, tumor tissue of mice was immunostained for CD31 and intravascular ICAM-1 as reported [17]. Arrows denote the position of tumor vessels based on CD31 staining; size bar, 100 µm.
In view of the chronically high levels of IL-6 trans-signaling in the tumor microenvironment and the refractory nature of tumor vessels in the face of potent inflammatory mediators, it came as a surprise when acute activation of IL-6 trans-signaling was found to promote the adhesive properties of the tumor endothelium (Figure 5A). In this regard, studies in tumor-bearing mice showed profound STAT3 activation in tumor-associated endothelial cells following systemic administration of H-IL-6 (Figure 5B). As STAT3 has been identified as a strong regulator of adhesion molecule expression in inflamed tissues [18,65], its activation in tumor vessels suggested that IL-6 trans-signaling could succeed where other inflammatory pathways failed. Remarkably, stimulation of IL-6 trans-signaling in tumor microvessels by H-IL-6 acted at multiple steps in the adhesion cascade to amplify interactions between circulating CD8+ effector T cells and the tumor endothelium (Figure 5A). Specifically, H-IL-6 enhanced both E- and P-selectin-dependent tethering and rolling interactions as well as ICAM-1-mediated firm arrest which was accompanied by strong upregulation of ICAM-1 density on tumor vessels (Figure 5C). Similar induction of cytotoxic T cell trafficking was observed in response to activation of endogenous IL-6 trans-signaling by systemic thermal therapy (i.e., using the same heating regimen shown to stimulate HEV adhesion described in Section 4.2) [17,56,57]. In proof-of-concept experiments, colorectal tumor explants from stage IV patients were shown to strongly increase ICAM-1 density on tumor vessels after ex-vivo treatment with H-IL-6, suggesting that the pro-adhesive functions of IL-6 trans-signaling are equally active in mouse and human tumor vessels [17]. The relevant cellular sources of IL-6 and sIL-6Rα controlling trafficking at tumor vascular loci remain to be fully identified. However, studies using bone marrow-chimeric mice demonstrated that the thermal response in tumor-bearing mice depends on IL-6 production by radiation-resistant, nonhematopoietic stromal cells (Figure 5A). These observations strongly implicate endothelial cells and/or cancer-associated fibroblasts as the major sources of IL-6 trans-signaling activity promoting T cell trafficking within the tumor microenvironment [17].
Observations that IL-6 trans-signaling increases CD8+ T cell trafficking following treatment with either H-IL-6 or systemic thermal therapy prompted further investigation into whether this activity translated into improved control of tumor growth. Interestingly, acute activation of IL-6 trans-signaling either by recombinant H-IL-6 or systemic thermal therapy had no effect on tumor growth, suggesting that the endogenous pool of newly recruited CD8+ T cells was insufficient to control tumor growth in aggressive murine cancer models [17]. To bypass impediments to T cell activation in situ in an immunuosuppressive cancer setting [1,3,4,6], the impact of IL-6-induced CD8+ T cell trafficking on tumors was examined using adoptive T cell transfer immunotherapy modeled after protocols currently in clinical use [17,84]. T cell-based adoptive cell transfer employs ex vivo activated and expanded T cells to augment the numbers of cytotoxic T cells in circulation [84]. While adoptive T cell transfer therapy offers great promise, durable response rates following treatment in patients are <25% [51,84]. The low baseline trafficking of CD8+ T cells in tumor vessels afforded a window of opportunity to enhance the efficacy of adoptive T cell transfer by using IL-6 trans-signaling to increase CD8+ T cell trafficking into tumors. This was shown to be the case in preclinical murine models where administration of H-IL-6 or systemic thermal therapy prior to adoptive transfer of tumor-specific effector CD8+ T cells was causally linked to enhanced apoptosis of tumor cell targets and overall delay in tumor growth [17]. These findings suggest new avenues for vascular-targeting strategies to synergize with immunotherapeutic interventions for improved patient outcomes.
The quiescent state of the tumor vascular endothelium coupled with the ability of an IL-6 trans-signaling mechanism driven by H-IL-6 or systemic thermal therapy to induce adhesion in the tumor microvasculature is counterintuitive given that IL-6 trans-signaling is already highly active in the tumor microenvironment. Results showing that transient augmentation of IL-6 trans-signaling does not accelerate tumor growth further suggests that it may be feasible to acutely harness the anti-tumor immune activities of IL-6 without also promoting tumor progression [17]. Although the underlying mechanistic basis for these confounding observations has not been fully resolved, a possible explanation is that tumor-associated endothelial cells are relatively insensitive to IL-6 trans-signaling activity and require higher amounts of cytokine than other cells in the tumor microenvironment. Thus, bolus injection of H-IL-6 could achieve high enough IL-6/sIL-6Rα concentrations to exceed the threshold needed to acutely activate the vasculature and induce adhesion molecule expression in an environment where cancer cells may already be maximally stimulated. Of related interest are findings that although thermal therapy does not alter the overall intratumoral concentration of IL-6 or sIL-6Rα, it causes upregulation of gp130 on the endothelial cells lining tumor microvessels which has been shown in inflammatory models to lower the activation threshold for IL-6 trans-signaling [17,85]. Taken together, findings that either H-IL-6 or thermal therapy can restrain tumor growth when combined with adoptive T cell immunotherapy suggest that acute activation of IL-6 in the tumor microenvironment could shift the balance between the darker and fairer faces of trans-signaling for therapeutic benefit.
5. Concluding Remarks
Studies discussed here and elsewhere in this issue detail the well-established malevolent face of IL-6 trans-signaling in the tumor microenvironment that promotes tumor cell proliferation, survival, angiogenesis, as well as evasion of immune surveillance. However, it is becoming increasingly apparent that the lesser known benevolent face of IL-6 trans-singling can counter-balance these effects by stimulating immune-mediated tumor control. Recent findings demonstrate that acute activation of IL-6 trans-signaling either through treatment with IL-6/sIL-6Rα fusion protein or stimulation of an endogenous IL-6 trans-signaling loop initiated by thermal therapy, exerts multiple effects on immune cells and the vasculature to orchestrate effective anti-tumor immunity. Trans-signaling has direct effects on lymphocytes, guiding their trafficking to lymph nodes as well as supporting their activation, proliferation, and polarization toward phenotypes that oppose the immunosuppressive tumor microenvironment. IL-6 also acts at discrete vascular sites; HEV and tumor endothelium, promoting their adhesive properties and enhancing trafficking of immune cells that deliver lethal hits to cancer cell targets within the complex tumor microenvironment. In light of the chronic IL-6 activity present in tumors, these results suggest that the tipping point between pro-tumor and anti-tumor effects likely represents a narrow target requiring that trans-signaling activity is delivered to the right cells, in the right place, at the right time in order to shift the balance toward the fairer face of IL-6 and tumor control.
Highlights.
IL-6 signaling supports tumor initiation and progression.
Activation of IL-6 trans-signaling improves T cell trafficking in lymphoid organs
IL-6 trans-signaling boosts T cell entry in tumors driving tumor immunity
Acknowledgments
We thank our many collaborators for their contributions to the development of this work, especially Dr. Qing Chen who initially characterized the roles of IL-6 trans-signaling in regulating trafficking in lymphoid organs and tumors. We thank Mark Bucsek and Maryann Mikucki for editorial comments, Amy Ku for assistance preparing the manuscript, and Dr. Jason Muhitch for providing the confocal micrograph depicting lymph node HEV. We also thank Karen Howard for original illustrations, and Robert Reincke for permission to adapt his sculpture, Multidimensional Multiples 2010, for this manuscript. This work was supported by the US National Institutes of Health (CA79765, 5T32 CA085183, and AI082039) and the Jennifer Linscott Tietgen Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drews J. Paul Ehrlich: magister mundi. Nat Rev Drug Discov. 2004;3(9):797–801. doi: 10.1038/nrd1498. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Res. 2012;72(10):2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117(12):3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelo LS, Talpaz M, Kurzrock R. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 2002;62(3):932–940. [PubMed] [Google Scholar]
- 16.Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88(6):1145–1156. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121(10):3846–3859. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardam TD, Zhou L, Appenheimer MM, Chen Q, Wang WC, Baumann H, et al. Regulation of a lymphocyte-endothelial-IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. Cytokine. 2007;39(1):84–96. doi: 10.1016/j.cyto.2007.07.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta. 2002;1592(3):251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 20.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21):2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 21.Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen KJ, Wollmer A, et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol. 1997;15(2):142–145. doi: 10.1038/nbt0297-142. [DOI] [PubMed] [Google Scholar]
- 22.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 23.Heikkila K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 25.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70(5):325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15(5):R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 34.Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, et al. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189(1):63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457(7226):200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilati C, Amessou M, Bihl MP, Balabaud C, Nhieu JT, Paradis V, et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med. 2011;208(7):1359–1366. doi: 10.1084/jem.20110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123(2):767–781. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barre B, Avril S, Coqueret O. Opposite regulation of myc and p21waf1 transcription by STAT3 proteins. J Biol Chem. 2003;278(5):2990–2996. doi: 10.1074/jbc.M210422200. [DOI] [PubMed] [Google Scholar]
- 39.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12(1):11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 40.Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, et al. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65(11):4673–4682. doi: 10.1158/0008-5472.CAN-04-3589. [DOI] [PubMed] [Google Scholar]
- 41.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23(20):3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 43.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 44.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118(10):3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183(2):937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 48.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 49.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 51.Mikucki ME, Fisher DT, Ku AW, Appenheimer MM, Muhitch JB, Evans SS. Preconditioning thermal therapy: Flipping the switch on IL-6 for anti-tumour immunity. Int J Hyperthermia. 2013;29(5):464–473. doi: 10.3109/02656736.2013.807440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dejean AS, Beisner DR, Ch'en IL, Kerdiles YM, Babour A, Arden KC, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10(5):504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 54.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179(4):2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 55.Appenheimer MM, Girard RA, Chen Q, Wang WC, Bankert KC, Hardison J, et al. Conservation of IL-6 trans-signaling mechanisms controlling L-selectin adhesion by fever-range thermal stress. Eur J Immunol. 2007;37(10):2856–2867. doi: 10.1002/eji.200636421. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7(12):1299–1308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 57.Chen Q, Wang WC, Bruce R, Li H, Schleider DM, Mulbury MJ, et al. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity. 2004;20(1):59–70. doi: 10.1016/s1074-7613(03)00358-3. [DOI] [PubMed] [Google Scholar]
- 58.Wang WC, Goldman LM, Schleider DM, Appenheimer MM, Subjeck JR, Repasky EA, et al. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol. 1998;160(2):961–969. [PubMed] [Google Scholar]
- 59.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97(9):2727–2733. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 60.Kraybill WG, Olenki T, Evans SS, Ostberg JR, O'Leary KA, Gibbs JF, et al. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumours: correlation with mouse models. Int J Hyperthermia. 2002;18(3):253–266. doi: 10.1080/02656730110116704. [DOI] [PubMed] [Google Scholar]
- 61.Chen Q, Fisher DT, Kucinska SA, Wang WC, Evans SS. Dynamic control of lymphocyte trafficking by fever-range thermal stress. Cancer Immunol Immunother. 2006;55(3):299–311. doi: 10.1007/s00262-005-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Q, Appenheimer MM, Muhitch JB, Fisher DT, Clancy KA, Miecznikowski JC, et al. Thermal facilitation of lymphocyte trafficking involves temporal induction of intravascular ICAM-1. Microcirculation. 2009;16(2):143–158. doi: 10.1080/10739680802353850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans SS, Bain MD, Wang WC. Fever-range hyperthermia stimulates alpha4beta7 integrin-dependent lymphocyte-endothelial adhesion. Int J Hyperthermia. 2000;16(1):45–59. doi: 10.1080/026567300285411. [DOI] [PubMed] [Google Scholar]
- 64.Evans SS, Schleider DM, Bowman LA, Francis ML, Kansas GS, Black JD. Dynamic association of L-selectin with the lymphocyte cytoskeletal matrix. J Immunol. 1999;162(6):3615–3624. [PubMed] [Google Scholar]
- 65.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 66.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 68.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4(6):579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 69.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 70.Hope JC, Cumberbatch M, Fielding I, Dearman RJ, Kimber I, Hopkins SJ. Identification of dendritic cells as a major source of interleukin-6 in draining lymph nodes following skin sensitization of mice. Immunology. 1995;86(3):441–447. [PMC free article] [PubMed] [Google Scholar]
- 71.Leal IS, Florido M, Andersen P, Appelberg R. Interleukin-6 regulates the phenotype of the immune response to a tuberculosis subunit vaccine. Immunology. 2001;103(3):375–381. doi: 10.1046/j.1365-2567.2001.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113(11):2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 74.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33(6):942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Andrian UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 1996;3(3):287–300. doi: 10.3109/10739689609148303. [DOI] [PubMed] [Google Scholar]
- 76.Repasky EA, Evans SS, Dewhirst MW. Temperature Matters! And Why It Should Matter to Tumor Immunologists. Cancer Immunol Res. 2013;1(4):210–216. doi: 10.1158/2326-6066.CIR-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukoc Biol. 2000;68(6):815–820. [PubMed] [Google Scholar]
- 78.Jiang Q, DeTolla L, van Rooijen N, Singh IS, Fitzgerald B, Lipsky MM, et al. Febrile-range temperature modifies early systemic tumor necrosis factor alpha expression in mice challenged with bacterial endotoxin. Infect Immun. 1999;67(4):1539–1546. doi: 10.1128/iai.67.4.1539-1546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carriere V, Colisson R, Jiguet-Jiglaire C, Bellard E, Bouche G, Al Saati T, et al. Cancer cells regulate lymphocyte recruitment and leukocyte-endothelium interactions in the tumor-draining lymph node. Cancer Res. 2005;65(24):11639–11648. doi: 10.1158/0008-5472.CAN-05-1190. [DOI] [PubMed] [Google Scholar]
- 80.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52(15):4265–4268. [PubMed] [Google Scholar]
- 81.Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996;2(9):992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- 82.Hellebrekers DM, Castermans K, Vire E, Dings RP, Hoebers NT, Mayo KH, et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. 2006;66(22):10770–10777. doi: 10.1158/0008-5472.CAN-06-1609. [DOI] [PubMed] [Google Scholar]
- 83.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klouche M, Bhakdi S, Hemmes M, Rose-John S. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J Immunol. 1999;163(8):4583–4589. [PubMed] [Google Scholar]
- 86.Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39(Web Server issue):W475–W478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]