Abstract
In this study, the in vitro effects of amodiaquine (AQ) monotherapy on the egg output of paired adult Schistosoma mansoni worms and their survival during in vitro culture were assessed. In addition, the gross morphological alterations of male and female worms caused by AQ were visually observed under a dissecting microscope. AQ significantly reduced the daily egg output of paired adult S. mansoni worms following incubation for 14 days at 1-5 µg/mL, but not at 0.5 µg/mL, compared with the control group. AQ also reduced the survival of male and female worms at concentrations of 2 and 5 µg/mL, respectively. Moreover, exposure to 5 µg/mL AQ caused severe swelling and/or localisation of black content in the body of all male and female worms within one or two days of incubation; subsequently, shrinkage in the male worms and elongation in the female worms were observed. The initial morphological alterations caused by AQ occurred along the intestinal tract of the male and female worms. To our knowledge, this is the first study to report not only the efficacy of AQ at concentrations lower than 5 µg/mL on paired adult S. mansoni worms, but also the effects of AQ on the intestinal tracts of worms in in vitro culture.
Keywords: Schistosoma mansoni, amodiaquine, antimalarial drug, antischistosomal drug
Schistosomiasis is still a major public health problem in many tropical and subtropical countries. An estimated 779 million people are at risk for the disease and 207 million people are infected worldwide (Steinmann et al. 2006). Praziquantel (PZQ) has become the drug of choice for the treatment of human schistosomiasis (WHO 2002). However, field studies indicate the presence of PZQ-resistant schistosomes in Senegal and Egypt (Guisse et al. 1997, Ismail et al. 1999). Concerns regarding the emergence of a tolerant and/or resistant schistosomal strain are increasing. Thus, research related to the development of a new schistosomicidal drug is required.
Recently, Keiser et al. (2009) reported the interesting finding that mefloquine (MQ), one of the antimalarial drugs currently in use, reduced the worm burden in mice infected with Schistosoma mansoni and Schistosoma japonicum. Furthermore, Xiao et al. (2009) confirmed the in vitro effects of MQ against juvenile and adult worms of S. japonicum. On the other hand, the antischistosomal activity of amodiaquine (AQ), another antimalarial drug that is currently in use, was not observed in the S. mansoni-infected mice after a single oral administration (Keiser et al. 2009). Mitsui and Aoki (2010), however, demonstrated that not only MQ, but also other antimalarial drugs, such as AQ, chloroquine (CQ) and primaquine (PQ), exhibited in vitro antischistosomal activity at a concentration of 10 µg/mL. Subsequently, the in vitro antischistosomal activity of PQ was confirmed at concentrations lower than 2 µg/mL (Holtfreter et al. 2011). Mitsui and Aoki (2010) observed that AQ exhibited higher in vitro antischistosomal activity than PQ at a concentration of 10 µg/mL and they cited AQ as a promising candidate for the treatment of schistosomiasis. Nevertheless, the in vitro antischistosomal activity of a relatively low concentration of AQ has not been investigated to date. This low concentration would correspond closely with the whole blood AQ concentration after administration of the drug at a dose of 10 mg/kg once daily for three days for the treatment of malaria (Winstanley et al. 1987, WHO 2001, Gitau et al. 2004).
The objective of this study was to evaluate the in vitro antischistosomal effects of AQ at concentrations of less than 5 µg/mL. The in vitro effects of the drug were examined with regard to the daily egg output of paired S. mansoni worms, survival and gross morphological alterations caused by the drug.
MATERIALS AND METHODS
Chemicals and media - AQ.2HCl was purchased from MP Biomedicals Inc (Fountain Parkway Solon, Ohio, USA) and diluted in deionised water to a concentration of 5 mg/mL (free base) as a stock solution. The stock AQ solution was then added to NCTC 135 medium (Sigma, St. Louis, Missouri, USA), containing a 1% solution of antibiotics (penicillin 5,000 units and streptomycin 5,000 mg/l; Gibco, Langley, Oklahoma, USA), at concentrations of 0.5-5 µg/mL.
Parasite strain - A Puerto Rican strain of S. mansoni (NIH-Sm-PR-1) was routinely maintained by passage through Mongolian gerbils (Merinoes unguiculatus; MGS/Sea, Kyudo Co, Ltd, Saga, Japan) and Biomphalaria glabrata snails (Newton's NIH Puerto Rican/Brazilian M-line) in the Animal Research Centre at the Institute of Tropical Medicine, Nagasaki University. At eight weeks post-infection, adult worms were collected by the perfusion technique as previously described (Smithers & Terry 1965) and washed twice with NCTC 135 medium.
The Animal Care and Use Committee, Nagasaki University, approved the experimental protocol and the experimental procedures were performed in accordance with the Guidelines for Animal Experimentation established by Nagasaki University.
Incubation of paired S. mansoni adult worms with AQ - The incubation of paired S. mansoni adult worms with AQ was conducted as previously described (Mitsui et al. 2009), except for the differences in the test drug and incubation period. Briefly, each pair of adult S. mansoni worms was preincubated for one day with 0.5 mL of NCTC 135 medium in a single well of a 24-well plate (Sumitomo Bakelite Co, Ltd, Osaka, Japan), in a 5% CO2 incubator at a temperature of 37ºC. Subsequently, 30 paired adult worms were randomly assigned to five groups: 0 (control), 0.5, 1, 2 or 5 µg/mL AQ. Then, each worm pair was transferred to a well containing 0.5 mL of NCTC 135 medium supplemented with 0, 0.5, 1, 2 or 5 µg/mL AQ. The plates were continuously incubated in the 5% CO2 incubator at 37ºC and the media alone or media supplemented with the drug was exchanged every day for 14 days. The number of eggs produced daily was counted and the morphological appearance of worms was also observed visually under a Nikon SMZ 800 stereoscopic microscope (Nikon Corporation, Tokyo, Japan).
The "dead or alive" status of adult worms was determined by the response of each worm to stimulation with a needle; that is, worms that failed to respond to needle stimulation were classified as "dead." Photographs of worms treated with the drug were obtained using a digital Senamal C-mount camera (Microscope Network, Saitama, Japan) mounted on the stereoscopic microscope.
Morphological investigation of intestinal tract alterations of male and female adult S. mansoni worms treated with AQ - Twelve paired adult S. mansoni worms were incubated under the same conditions described above and divided into two groups. The six pairs of adult worms were incubated either with 0 (control) or 5 µg/mL AQ for two days, with daily changes of the media alone or media supplemented with the drug. Then, the living male and female worms were individually mounted on a glass slide (76 x 26 x 0.9 ~1.2 mm; Matsunami Glass Industries, Ltd, Osaka, Japan) with two droplets of NCTC 135 medium, sandwiched with a coverslip (18 x 18 mm, Nº1, Matsunami Glass Industries, Ltd, Osaka, Japan) and immediately observed under a stereoscopic microscope equipped with a digital camera.
Data analysis - The data were analysed using Epi-Info software (Canters for Disease Control and Prevention, Atlanta, Georgia, USA). The daily egg output per female adult worm was expressed as the arithmetic mean (± standard error). The Kruskal-Wallis or Mann-Whitney U test was used to compare daily egg output between the AQ-treated and control groups. A log-rank test was used to compare the survival time of worms between the AQ-treated and control groups.
RESULTS
Effect of AQ on the daily egg output of paired S. mansoni adult worms - The in vitro effects of AQ on the daily egg output of paired adult worms at different concentrations are shown in Table I. The mean daily egg output during the one-day preincubation period in the control group did not differ significantly from that observed in the four AQ-treated groups. On the first day of exposure, AQ concentrations of 0.5, 1, 2 and 5 µg/mL did not significantly reduce the mean daily egg output compared with that of the control group. However, on days 2 and 3 after exposure of the worms to AQ, the mean daily egg output was significantly lower in the 1, 2 and 5 µg/mL AQ-treated groups than in the control group. Throughout the incubation period of seven days, the mean daily egg output of the 0.5 µg/mL AQ-treated group was similar to that of the control group.
TABLE I. Effect of amodiaquine on the daily egg output of six paired Schistosoma mansoni adult worms within seven days.
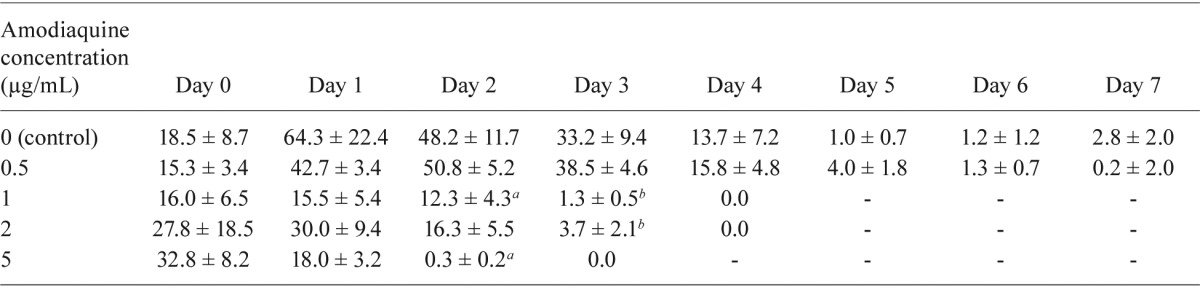
: p ≤ 0.05;
: p ≤ 0.01, compared with the control group. Statistical analysis by Manny-Whiteny U test.
Effect of AQ on the survival of paired S. mansoni adult worms - Table II shows the effect of continuous in vitro incubation with different concentrations of AQ on the survival of paired adult S. mansoni worms. None of the worms died in the control group during the 14-day incubation period. On the other hand, all of the male and female worms in the 5 µg/mL AQ group died within eight days. In the 2 µg/mL AQ group, four of the six males and four of the six females died before the end of the 14-day incubation period. No worm deaths were observed in the 0.5 or 1 µg/mL AQ groups during the 14-day incubation period.
TABLE II . Effect of amodiaquine on the survival of six paired Schistosoma mansoni adult worms within 14 days.
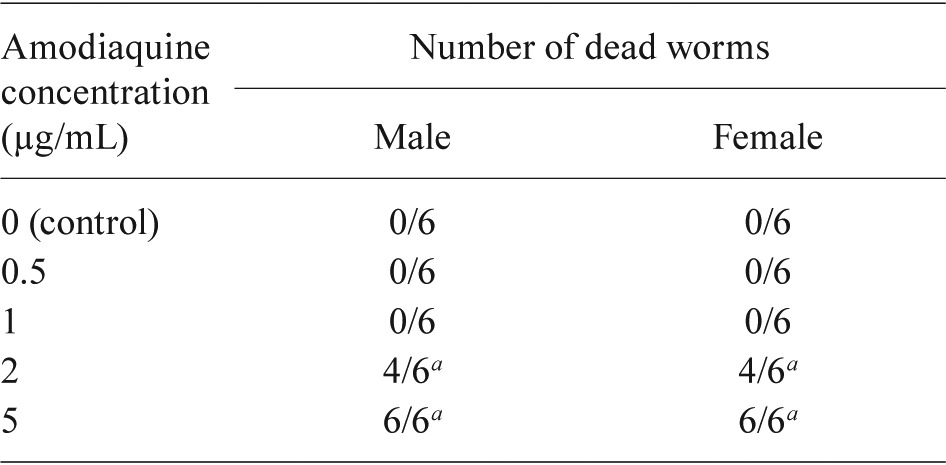
: statistically significant vs. control at p ≤ 0.01 by a log-rank test.
Gross alterations of S. mansoni adult worms caused by AQ - The gross morphological appearance of paired adult worms exposed to AQ was observed daily under a dissecting microscope. When paired adult worms were consecutively cultivated in media alone (control group), the black content in the body of all male worms gradually disappeared over a period of three days (Fig. 1A-C). The black content in the body of separated female worms also disappeared. Subsequently, both male and female worms shrank in size after a seven-day incubation period (Fig. 1D). On the other hand, when paired adult worms were exposed to 5 µg/mL AQ for one or two days, severe swelling and localisation of black content appeared in the body of the male worms (Fig. 1F). On day 3 after exposure to 5 µg/mL AQ, the male worms appeared slightly shrunken and exhibited visible localisation of black content, while the female worms were elongated and exhibited a similar localisation of black content (Fig. 1G). On day 7 after exposure to 5 µg/mL AQ, the male worms had shrunken further and simultaneously turned opaque with a damaged surface; in contrast, the female worms had elongated further and eroded and their surface had become sticky (Fig.1H). Finally, all of the male and female worms died within eight days of incubation.
Fig. 1. effect of amodiaquine on gross morphological alterations in male and female adult worms of Schistosoma mansoni. Paired adult worms were incubated in media alone for zero (A), one (B), three (C) and seven (D) days (control group), while paired adult worms were incubated in media with 5 µg/mL amodiaquine for zero (E), one (F), three (G) and seven (H) days. Arrows indicate localization of black content. The symbols ♂ and ♀ indicate male and female worms, respectively.
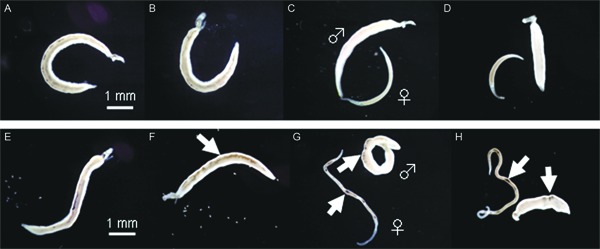
The morphological alterations observed (severe swelling and/or localisation of black content) also appeared in all of the male and female worms at low concentrations of 1 and 2 µg/mL AQ during the incubation periods of five-seven and three-four days, respectively. At 0.5 µg/mL, the specific morphological alterations appeared in all males as early as 10 days of incubation, but they were not observed in any of the females during the 14-day incubation period. Thus, the incubation time at which AQ caused the specific morphological alterations in male and female worms was closely related to the drug concentration used.
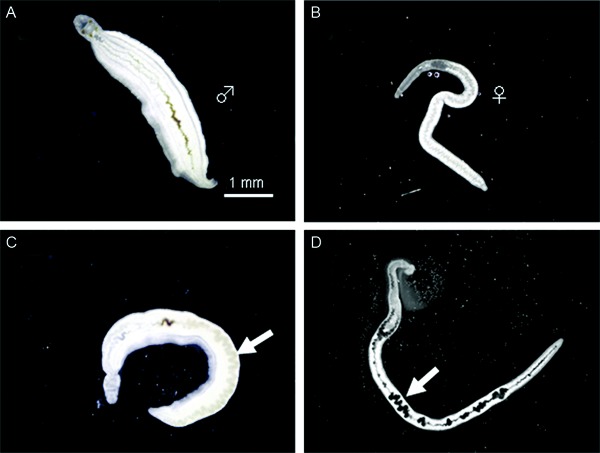
Effects of AQ on the intestinal tracts of adult male and female S. mansoni worms - Two days after the paired adult S. mansoni worms were continuously incubated in a drug-free medium (control group) or with 5 µg/mL AQ, a small amount of black content lingering along the intestinal tract of males (Fig. 2A) was observed in the control group. Meanwhile, the intestinal tracts of the female worms appeared empty (Fig. 2B). In contrast, severe swelling and localisation of black content were visible in the male and female worms after two days of exposure to 5 µg/mL AQ (Fig. 2C,).
Fig. 2. effect of amodiaquine on morphological alterations of the intestinal tract of male and female adult worms of Schistosoma mansoni. Paired adult male (A) and female (B) worms were incubated in media alone for two days (control group), while another pair of adult male (C) and female (D) worm was incubated with amodiaquine at a concentration of 5 µg/mL for two days. Each living worm was placed between a slide glass with medium and a glass coverslip and imme-diately observed. Arrows indicate the severe swelling of the intestinal tract of the male (♂) and localization of black content along the intestinal tract of the female (♀) worm.
DISCUSSION
Although monotherapy with artesunate (ART) in Schistosoma haematobium-infected children yields a low cure rate of 21% (Keiser et al. 2010), a high cure rate of 100% was obtained with the combination therapy of ART and AQ (Boulanger et al. 2007). These results suggest that AQ strongly exhibits antischistosomal activity. After oral administration at a dose of 10 mg/kg once daily for three days for the treatment of malaria in humans (WHO 2001), the blood concentration of AQ and its major metabolite N-desethylamodiaquine (DEAQ) may reach a level close to 1 µg/mL (Winstanley et al. 1987, Gitau et al. 2004). Thus, it was surmised that AQ exerts antischistosomal activity at this concentration. However, the in vitro antischistosomal effect of AQ has not been further investigated at this concentration, although Mitsui and Aoki (2010) reported that AQ alone exerted an in vitro antischistosomal effect at a concentration of 10 µg/mL. An investigation of the antischistosomal activity of AQ at the low concentration of 1 µg/mL is very important to assess whether AQ alone exerts antischistosomal activity at the regular dose used for the treatment of malaria. Therefore, the aim of this study was to investigate the schistosomicidal effect of AQ on adult S. mansoni worms at the relatively low concentrations of 0.5-5 µg/mL.
When S. mansoni worm pairs were exposed to AQ at concentrations of 1, 2 and 5 µg/mL, the daily egg output was significantly lower than that of the control group after two or three days of incubation. Meanwhile, the daily egg output of the 0.5 µg/mL AQ group was similar to that of the control group. Thus, the minimum concentration of AQ that was effective against the daily egg output of S. mansoni worm pairs is presumed to lie between 0.5-1 µg/mL. Furthermore, the results of the present study showed that AQ reduced the survival time of adult worms at concentrations of 2 and 5 µg/mL during the 14-day incubation period. In addition, AQ caused specific morphological alterations in all of the male and female adult worms of S. mansoni at concentrations of 1, 2 and 5 µg/mL during the incubation periods of five-seven, three-four and one-two days, although these alterations were delayed in the group treated with 0.5 µg/mL AQ. The direct effect of AQ on the daily egg output and morphological alterations of paired adult worms seemed to depend on the concentration and duration of exposure to the drug.
The effect of a drug is usually attributable to its pharmacokinetics in human subjects. The in vitro effects of AQ on adult S. mansoni worms demonstrated in the present study may not entirely reflect the in vivo effects of AQ. In fact, after oral administration, AQ is rapidly and predominantly metabolised to DEAQ (Churchill et al. 1985, Winstanley et al. 1987). Although both AQ and DEAQ exhibit antimalarial activity, the activity of DEAQ is approximately 3.5 times lower than that of AQ (Churchill et al. 1985, Childs et al. 1989). It follows, therefore, that the antischistosomal activity of DEAQ is also lower than that of AQ. Furthermore, during the regular treatment of malaria in an adult subject, the average concentration of DEAQ in whole blood reached 0.56 ± 0.07 µg/mL at 2.2 ± 0.5 h and gradually decreased to approximately 0.2 µg/mL (Winstanley et al. 1987) within 24 h. Thus, the standard dose of AQ for malaria (10 mg/kg of body weight, once daily for 3 days) is probably not sufficient to produce therapeutic effects in the treatment of schistosomiasis.
Schistosomes ingest the host red blood cells and degrade haemoglobin by converting toxic haem into haemozoin (Lawrence 1973, Brindley et al. 1997). Oliveira et al. (2004) showed that CQ, one of the 4-aminoquinoline derivatives similar in structure to AQ, exerted an effect on schistosomes by inhibiting the formation of haemozoin, a detoxification product of free haem. Similarly, it is thought that free haem produced by AQ may be responsible for damaging reproductive organs or killing worms. The free haem may also simultaneously damage the intestinal tract of male and female worms and suspend its rhythmic and peristaltic movement, resulting in severe swelling of the intestinal tract and/or a striking localisation of black content in the intestinal tract of worms as shown in Fig. 2C, D. These morphological alterations in male and female worms incubated with AQ had a very specific appearance. Although Holtfreter et al. (2011) did not report the effect of PQ on the intestinal tract of male and female worms, PQ, which is similar in structure to AQ, most likely also causes morphological alterations in the intestinal tract of male and female worms.
Some field trials have revealed a strong antischistosomal effect of combination therapies consisting of ART and current antimalarial drugs (Boulanger et al. 2007, Mohamed et al. 2009, Sissoko et al. 2009, Keiser et al. 2010), indicating that ART exhibits antischistosomal activities that are synergistic with current antimalarial drugs. Because ACT with AQ (WHO 2006) is currently recommended as a first-line treatment for malaria, the combination therapy using ART and AQ for the treatment of malaria in schistosomiasis-endemic areas may elicit an additional therapeutic effect on schistosomiasis. Thus, the present findings are noteworthy and can be expected to contribute to the evaluation of the therapeutic effects of the combination therapy on schistosomiasis in field trials. No serious side effects of AQ have been registered to date (D'Alessandro & ter Kuile 2006), but minor side effects are relatively frequent in pregnant women treated with AQ alone. Thus, care should be exercised in the use of AQ alone or in combination with ART for the treatment of schistosomiasis.
Acknowledgments
To Dr Yoshiki Aoki, Graduate School of International Health Development, Nagasaki University, for assisting in the revision of the paper.
REFERENCES
- Boulanger D, Dieng Y, Cisse B, Remoue F, Capuano F, Dieme JL, Ndiaye T, Sokhna C, Trape JF, Greenwood B, Simondon F. Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks. Trans R Soc Trop Med Hyg. 2007;101:113–116. doi: 10.1016/j.trstmh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, Kalinna BH, Dalton JP, Day SR, Wong JY, Smythe ML, McManus DP. Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol. 1997;89:1–9. doi: 10.1016/s0166-6851(97)00098-4. [DOI] [PubMed] [Google Scholar]
- Childs GE, Boudreau EF, Milhous WK, Wimonwattratee T, Pooyindee N, Pang L, Davidson DE., Jr A comparison of the in vitro activities of amodiaquine and desethylamodiaquine against isolates of Plasmodium falciparum . Am J Trop Med Hyg. 1989;40:7–11. doi: 10.4269/ajtmh.1989.40.7. [DOI] [PubMed] [Google Scholar]
- Churchill FC, Patchen LC, Campbell CC, Schwartz IK, Nguyen-Dinh P, Dickinson CM. Amodiaquine as a prodrugimportance of metabolite(s) in the antimalarial effect of amodiaquine in humans. Life Sci. 1985;36:53–62. doi: 10.1016/0024-3205(85)90285-1. [DOI] [PubMed] [Google Scholar]
- D'Alessandro U, ter Kuile FO. Amodiaquine, malaria, pregnancy: the old new drug. Lancet. 2006;368:1306–1307. doi: 10.1016/S0140-6736(06)69532-9. [DOI] [PubMed] [Google Scholar]
- Gitau EN, Muchohi SN, Ogutu BR, Githiga IM, Kokwaro GO. Selective and sensitive liquid chromatographic assay of amodiaquine and desethylamodiaquine in whole blood spotted on filter paper. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:173–177. doi: 10.1016/j.jchromb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Guisse F, Polman K, Stelma FF, Mbaye A, Talla I, Niang M, Deelder AM, Ndir O, Gryseels B. Therapeutic evaluation of two different dose regimens of praziquantel in a recent Schistosoma mansoni focus in Northern Senegal. Am J Trop Med Hyg. 1997;56:511–514. doi: 10.4269/ajtmh.1997.56.511. [DOI] [PubMed] [Google Scholar]
- Holtfreter MC, Loebermann M, Klammt S, Sombetzki M, Bodammer P, Riebold D, Kinzelbach R, Reisinger EC. Schistosoma mansoni: schistosomicidal effect of mefloquine and primaquine in vitro. Exp Parasitol. 2011;127:270–276. doi: 10.1016/j.exppara.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. Mefloquine - an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3: doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, N'Guessan NA, Adoubryn KD, Silué KD, Vounatsou P, Hatz C, Utzinger J, N'Goran EK. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50:1205–1213. doi: 10.1086/651682. [DOI] [PubMed] [Google Scholar]
- Lawrence JD. The ingestion of red blood cells by Schistosoma mansoni . J Parasitol. 1973;59:60–66. [PubMed] [Google Scholar]
- Mitsui Y, Aoki Y. In vitro effects of current antimalarial drugs on the survival of paired Schistosoma mansoni adult worms and their egg production. Trop Med Health. 2010;38:69–73. [Google Scholar]
- Mitsui Y, Miura M, Aoki Y. In vitro effects of artesunate on the survival of worm pairs and egg production of Schistosoma mansoni . J Helminthol. 2009;83:7–11. doi: 10.1017/S0022149X08070235. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Mahgoub HM, Magzoub M, Gasim GI, Eldein WN, Ahmed Ael A, Adam I. Artesunate plus sulfadoxine/pyrimethamine versus praziquantel in the treatment of Schistosoma mansoni in eastern Sudan. Trans R Soc Trop Med Hyg. 2009;103:1062–1064. doi: 10.1016/j.trstmh.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Oliveira MF, d'Avila JC, Tempone AJ, Soares JB, Rumjanek FD, Ferreira-Pereira A, Ferreira ST, Oliveira PL. Inhibition of heme aggregation by chloroquine reduces Schistosoma mansoni infection. J Infect Dis. 2004;190:843–852. doi: 10.1086/422759. [DOI] [PubMed] [Google Scholar]
- Sissoko MS, Dabo A, Traoré H, Diallo M, Traoré B, Konaté D, Niaré B, Diakité M, Kamaté B, Traoré A, Bathily A, Tapily A, Touré OB, Cauwenbergh S, Jansen HF, Doumbo OK. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS ONE. 2009;4: doi: 10.1371/journal.pone.0006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- WHO - World Health Organization . The use of antimalarial drugs: Report of an informal consultation. 2001. Available from: rollbackmalaria.org/cmc_upload/0/000/014/923/use_of_antimalarials.pdf. [Google Scholar]
- WHO - World Health Organization Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Se. 2002;912:1–57. [PubMed] [Google Scholar]
- WHO - World Health Organization . Guidelines for the treatment of malaria. 2006. Available from: whqlibdoc.who.int/publications/2006/9241546948_eng.pdf. [Google Scholar]
- Winstanley P, Edwards G, Orme M, Breckenridge A. The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol. 1987;23:1–7. doi: 10.1111/j.1365-2125.1987.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SH, Mei JY, Jiao PY. The in vitro effect of mefloquine and praziquantel against juvenile and adult Schistosoma japonicum . Parasitol Res. 2009;106:237–246. doi: 10.1007/s00436-009-1656-x. [DOI] [PubMed] [Google Scholar]


