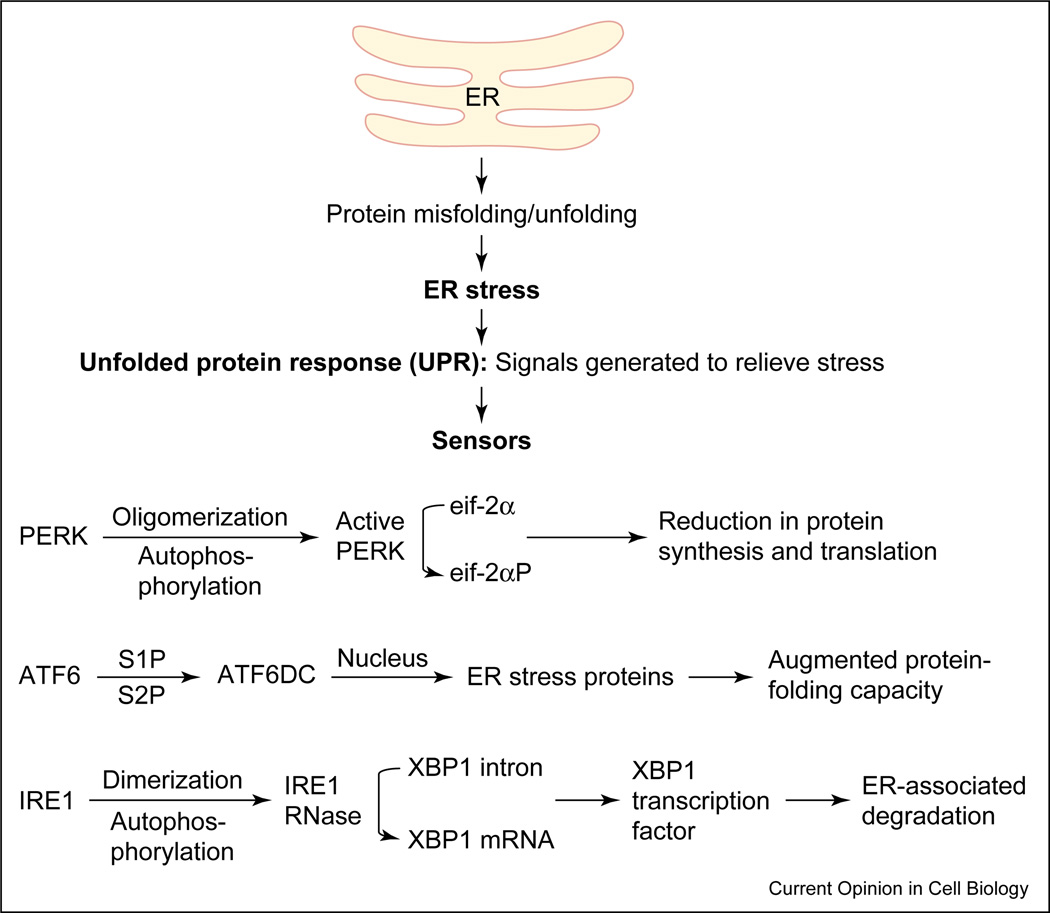
Figure 1.
Accumulation of misfolded proteins in the ER can disrupt ER function resulting in ‘ER stress’. The ER responds by triggering specific signaling pathways including the UPR. The UPR is coordinately regulated by the three proximal sensors, IRE1, PERK and ATF6. The activation of all three proximal sensors results in reduction in the amount of new protein translocated into the ER lumen, increased degradation of ER-localized proteins and increased protein folding capacity of the ER. ATF6DC represents the 50kD cytosolic bZIP-containing fragment that translocates to the nucleus to activate transcription.