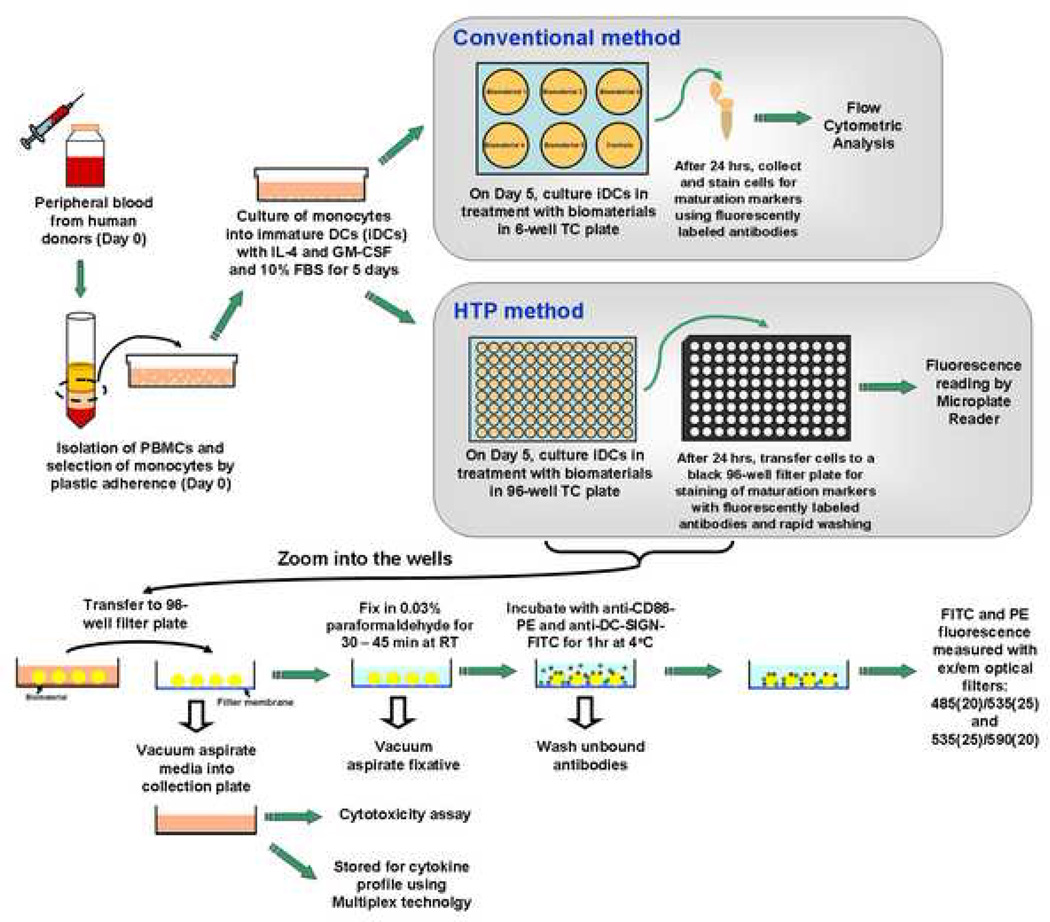
Figure 1.
A schematic of the conventional method and the HTP method for analyzing DC response to biomaterials. For both of the analysis methods, DCs were derived from human peripheral blood mononuclear cells (PBMCs) using the same procedures until day 5. On day 5, for the conventional method, DCs were treated with biomaterials in a 6-well plate for 24 hours. The cells after treatment are then collected and stained, and flow cytometry is performed to analyze the cell surface marker expression. In contrast, for the HTP method, DCs are treated with biomaterials in a 96-well plate for 24 hours. On day 6, DCs are transferred to a 96-well filtration plate, fixed and then stained with anti-CD86-PE and anti-DC-SIGN-FITC antibodies for 1 hour and washed. The relative fluorescence intensity is subsequently measured by a Tecan Infinite F500 microplate reader. Simultaneously, the cell culture supernatants from each well can be aspirated into a collection plate and tested for cytotoxicity and stored for cytokine profiling using Multiplex technology.