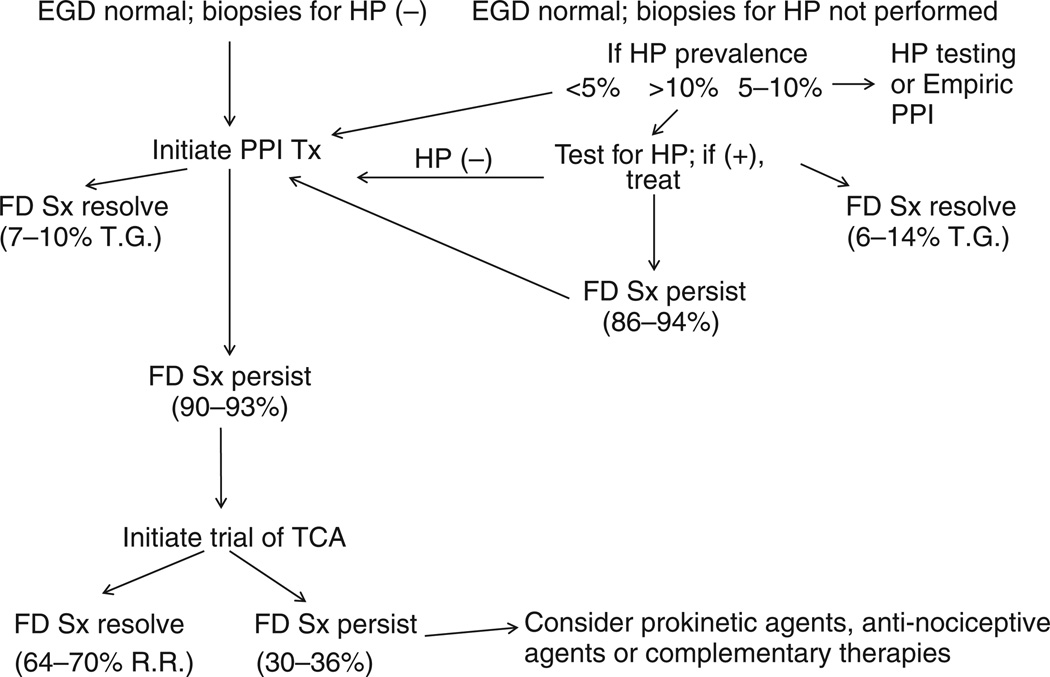
Figure 1.
Proposed treatment algorithm for FD. In this algorithm the patient with dyspepsia undergoes an upper endoscopy which, by definition, has to be grossly normal. If biopsies for Helicobacter pylori (HP) were performed and were negative (upper left corner) then the patient should be treated with a daily proton pump inhibitor (PPI). If symptoms (Sx) do not improve after 4–8 weeks, a therapeutic trial with a TCA should be initiated (the percentage of patients with FD symptom resolution is shown in parentheses). If upper endoscopy is grossly normal but gastric biopsies were not obtained for H. pylori, then the algorithm should begin with an assessment of H. pylori prevalence (upper right side). If present, H. pylori should be treated and the occasional patient with H. pylori will experience FD symptom resolution. If the patient is H. pylori-negative, then PPI therapy should be initiated (upper middle of diagram). The percentage of H. pylori-negative FD patients previously treated with a PPI and then a TCA, who will improve with an anti-nociceptive agent or CAM, is unknown. Therapeutic Gain (T.G.) refers to the reported symptom improvement rate above the placebo response (i.e. not including the placebo response). The overall Response Rate (R. R.) refers to the overall response rate which includes the placebo response. For example, on the right hand side of the diagram, the therapeutic gain (T.G.) of treating an FD patient who is H.P (+) has been reported as 6–14%. Alternatively, this portion of the figure could have been labelled using the overall response rate (R.R.) for FD symptom improvement in a patient treated for HP, which is approximately 31–39%, as this includes the placebo response rate which is approximately 25%.