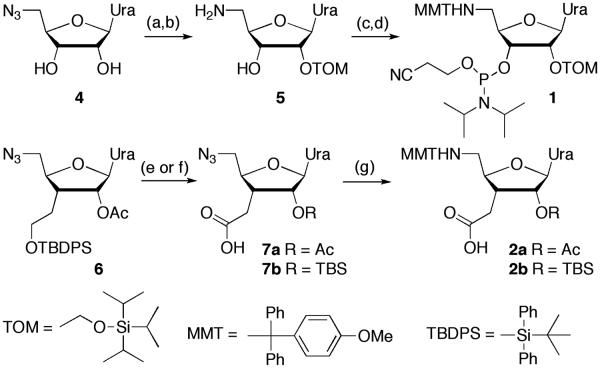
Scheme 1.
Synthesis of protected uridine 5′-amino 3′-C-carboxylic acids. Steps: (a) Bu2SnCl2, diisopropylethylamine, dichloroethane, rt, 1 h, then TOM-Cl, 80 °C, 0.5 h, 36%; (b) H2S gas, pyridine/water, 4:1, 1h, then stirred overnight, 88%; (c) methoxytrityl chloride, pyridine, rt, overnight, 77%; (d) 2-cyanoethyl N,N-diisopropylchlorophosphoramidite, diisopropylethylamine, CH2Cl2, rt, 14 h, 61%; (e) ref. 18; (f) ref. 19; (g) ref. 18.