Figure 11.
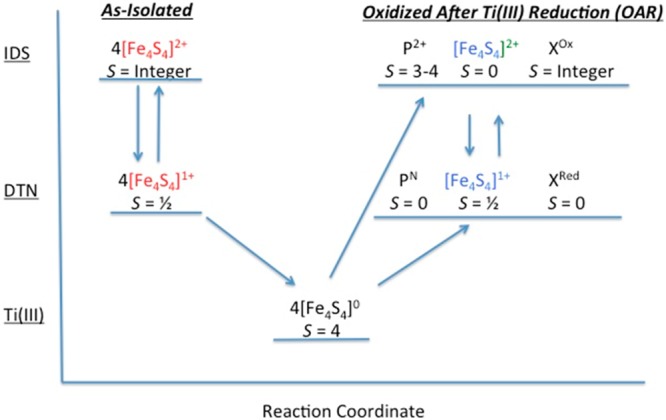
Flow diagram showing the spin states of the different FeS clusters in IDS, DTN, and Ti(III) citrate from different preparations. The different states of the clusters are placed along a reaction coordinate to show the irreversibility of the system following reduction by Ti(III) citrate to the all-ferrous state and subsequent oxidation with IDS or DTN. Note the different colors of the [Fe4S4] clusters in the as-isolated protein (red), the Ti(III) citrate-reduced protein (black), and the OAR protein (blue). These different colors are used to signify that these [Fe4S4]-like clusters differ from one another [i.e., the clusters in the as-isolated protein are paramagnetic in the 2+ state, the all-ferrous clusters in the Ti(III) citrate-reduced protein cannot be converted back to the cluster type of the as-isolated protein, and the OAR clusters are diamagnetic in the 2+ state].
