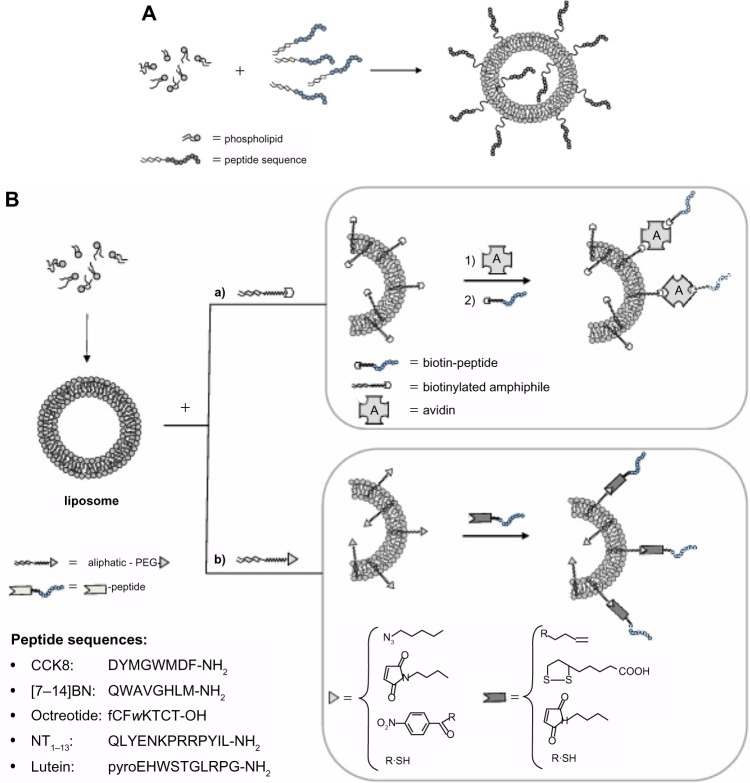
Figure 1.
Introduction of bioactive peptides on aggregate surfaces.
Notes: (A) The bioactive peptide may be introduced on the aggregate surface directly during nanostructure preparation by coupling the peptide to an amphiphilic moiety according to a pre-functionalization strategy; with this approach, however, the bioactive peptide is displayed on the external liposome surface as well as in the inner compartment. (B) Alternatively, peptide introduction can be performed after nanostructures have been obtained, according to a post-functionalization strategy. For the second approach, peptide coupling after liposome or nanoparticle preparation involves the introduction of suitable activated functional groups onto the external side of liposomes or nanoparticles for covalent or non-covalent peptide binding. To guarantee correct orientation of the targeting ligand, biorthogonal and site-specific surface reactions are necessary. Functional groups commonly used are: 1) amine for the amine-N-hydroxysuccinamide coupling method, 2) maleimide for Michael addition, 3) azide for Cu(I)-catalized Huisgen cycloaddition (CuAAC), 4) biotin for non-covalent interaction with avidin or triphosphines for Staudinger ligation, and hydroxylamine for oxime bond. In the inset are reported the peptide sequences.
Abbreviations: BN, bombesin; CCK8, cholecystokinin-8; NT, neurotensin; PEG, polyethylene glycol.