Abstract
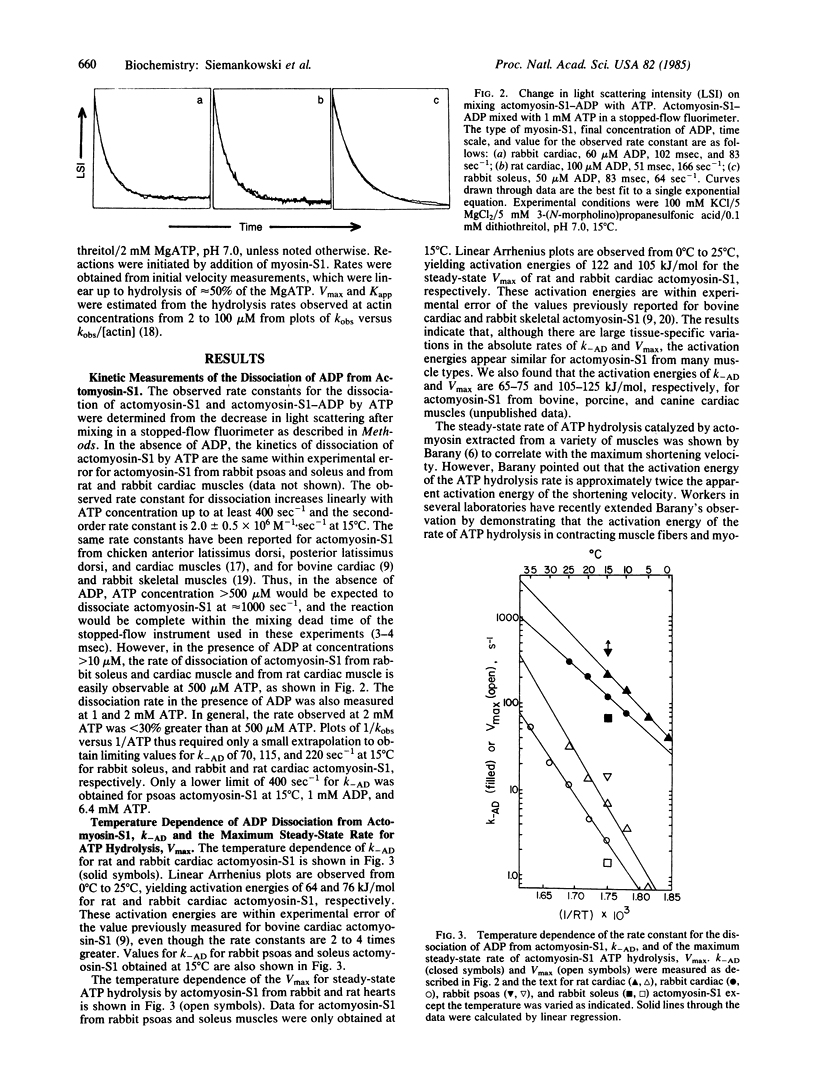
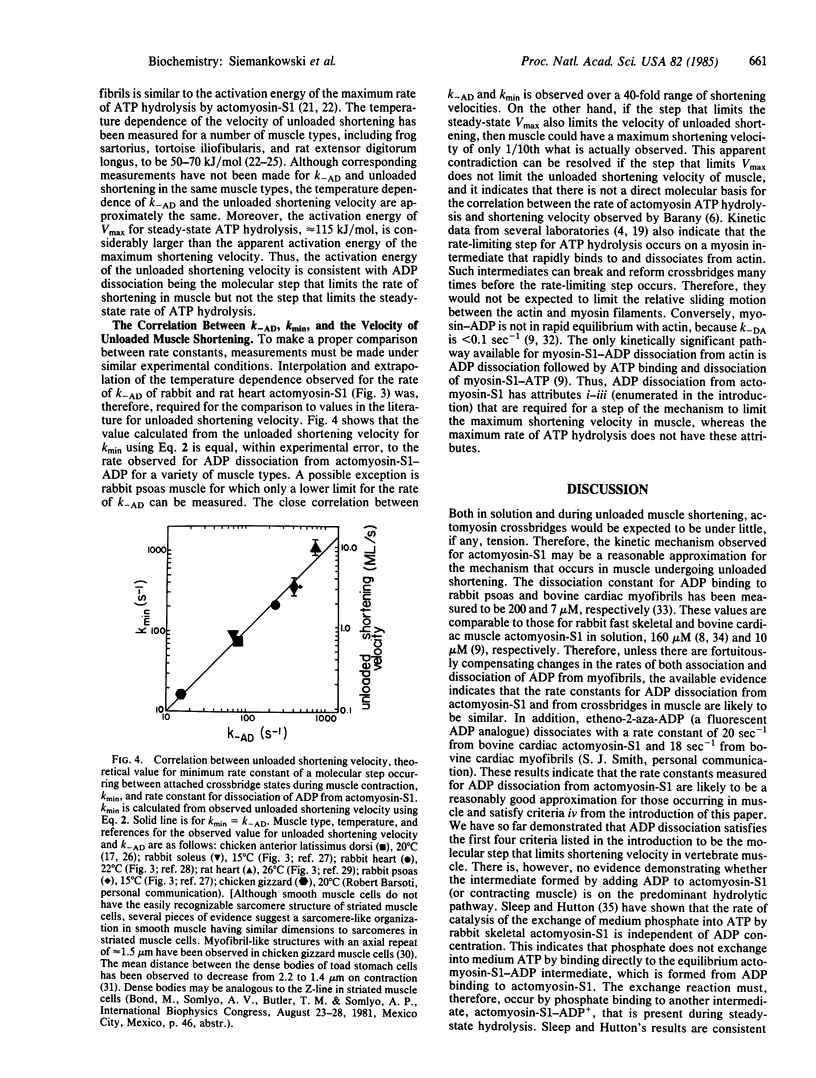
The rate constant for dissociation of ADP from actomyosin subfragment 1 (S1) has been measured in this laboratory and elsewhere for a variety of vertebrate muscle types. We have made the following observations: (i) In solution, the dissociation of ADP from actomyosin-S1 limits the rate of dissociation of actomyosin-S1-ADP by ATP and, presumably, also limits the rate of crossbridge detachment in contracting muscle. (ii) For muscle types in which the rate of ADP dissociation from actomyosin-S1 is slow enough to measure using stopped-flow methods, the rate constants are nearly the same as the theoretical value for the minimum allowable rate constant for dissociation of an attached crossbridge. Therefore, ADP dissociation is sufficiently slow to be the molecular step that limits the maximum shortening velocity of these muscles. (iii) Variation with muscle type of the rate constant for ADP dissociation may be a general phylogenetic mechanism for regulating shortening velocity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby R. M., Pepe F. A. Striated myofibrils in anti-myosin stained, isolated chicken gizzard smooth muscle cells. Histochemistry. 1978 Dec 1;58(3):219–235. doi: 10.1007/BF00495721. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch W. W., Moos C. Effect of temperature on actin activation of heavy meromyosin ATPase. Biochim Biophys Acta. 1971 May 11;234(2):183–189. doi: 10.1016/0005-2728(71)90073-9. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield S. P. The mechanical properties and heat production of chicken latissimus dorsi muscles during tetanic contractions. J Physiol. 1971 Dec;219(2):281–302. doi: 10.1113/jphysiol.1971.sp009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Fay F. S., Fujiwara K., Rees D. D., Fogarty K. E. Distribution of alpha-actinin in single isolated smooth muscle cells. J Cell Biol. 1983 Mar;96(3):783–795. doi: 10.1083/jcb.96.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S., Olajos M., Friedman H., Roeske W. R., Morkin E. Left ventricular performance in conscious thyrotoxic calves. Am J Physiol. 1982 Jan;242(1):H113–H121. doi: 10.1152/ajpheart.1982.242.1.H113. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E. Reaction free energy surfaces in myosin-actin-ATP systems. Biochemistry. 1976 Apr 20;15(8):1629–1635. doi: 10.1021/bi00653a006. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Adams P. H. ADP binds similarly to rigor muscle myofibrils and to actomyosin-subfragment one. FEBS Lett. 1984 Aug 20;174(1):11–14. doi: 10.1016/0014-5793(84)81067-4. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol. 1980 Jun 5;139(4):573–600. doi: 10.1016/0022-2836(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Maughan D., Low E., Litten R., 3rd, Brayden J., Alpert N. Calcium-activated muscle from hypertrophied rabbit hearts. Mechanical and correlated biochemical changes. Circ Res. 1979 Feb;44(2):279–287. doi: 10.1161/01.res.44.2.279. [DOI] [PubMed] [Google Scholar]
- Moss R. L. The effect of calcium on the maximum velocity of shortening in skinned skeletal muscle fibres of the rabbit. J Muscle Res Cell Motil. 1982 Sep;3(3):295–311. doi: 10.1007/BF00713039. [DOI] [PubMed] [Google Scholar]
- Pollack G. H., Krueger J. W. Sarcomere dynamics in intact cardiac muscle. Eur J Cardiol. 1976 May;4 (Suppl):53–65. [PubMed] [Google Scholar]
- Siemankowski R. F., White H. D. Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J Biol Chem. 1984 Apr 25;259(8):5045–5053. [PubMed] [Google Scholar]
- Sleep J. A., Hutton R. L. Exchange between inorganic phosphate and adenosine 5'-triphosphate in the medium by actomyosin subfragment 1. Biochemistry. 1980 Apr 1;19(7):1276–1283. doi: 10.1021/bi00548a002. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Gordon T., Shriver J. Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J. 1982 Nov;40(2):97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Bailin G., Bárány K., Bárány M. Proteolytic fragmentation of bovine heart heavy meromyosin. Biochemistry. 1969 Dec;8(12):4842–4850. doi: 10.1021/bi00840a029. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Transient kinetics of adenosine 5'-diphosphate and adenosine 5'-(beta, gamma-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry. 1982 Mar 16;21(6):1284–1294. doi: 10.1021/bi00535a028. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- White H. D. Special instrumentation and techniques for kinetic studies of contractile systems. Methods Enzymol. 1982;85(Pt B):698–708. doi: 10.1016/0076-6879(82)85057-x. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]