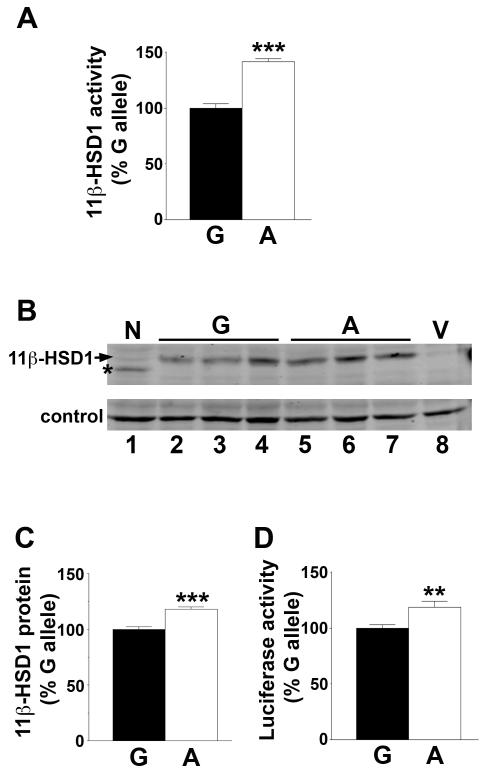
Figure 3. The A allele of SNP rs13306421 is associated with higher enzyme activity.
(A) Site-directed mutagenesis was used to change G at −2 of 11β-HSD1 (with respect to the ATG translation start) to A. 11β-Reductase activity was measured by conversion of 200nM [3H]-cortisone to cortisol following co-transfection of CHO cells with expression plasmids encoding H6PD and either the G (black bar) or A allele (white bar) of 11β-HSD1. 11β-HSD1 activity was normalised between experiments by arbitrarily setting the mean conversion of the G allele plasmids to 100% for each experiment.
(B) Representative western blot showing levels of 11β-HSD1 protein encoded by 3 independent preparations of pCMV-h11β-HSD1(G) (lanes 2–4), pCMV-h11β-HSD1(A) (lanes 5–7), 11β-HSD1 N162Q (lane 1; a mutation at one of the glycosylation sites of human 11β-HSD1) or pcDNA3.1 vector (V, lane 8). 50μg protein was loaded per lane. The membrane was incubated with sheep anti-human 11β-HSD1 antibody. A cross-reacting band of ~58kDa, present in untransfected cells, was used as a convenient loading control.
(C) Quantification of immunoblot shown in (B). Levels of 11β-HSD1 are expressed relative to the loading control, with the G allele arbitrarily set to 100%. Data are means ± SEM from 3 independent transfections, with 3 independent plasmid preps of either the A allele or the G allele. Significance was tested with unpaired Student’s t-test; ***, p<0.001.
(D) The A allele directs higher expression of a heterologous protein, luciferase. The leader sequence of luciferase (to the ATG translation start site) was replaced with the 11β-HSD1 leader sequence; either the A or G allele. Luciferase activity was measured in transfected CHO cells. To normalise between experiments the mean luciferase activity of the G allele plasmid was arbitrarily set to 100% for each experiment.
Data are means ± SEM from 4–6 independent transfections, each carried out in triplicate and with 3 independent plasmid preps of either the A allele or the G allele. Significance was tested with unpaired Student’s t-test; **, p<0.01; ***, p<0.001.