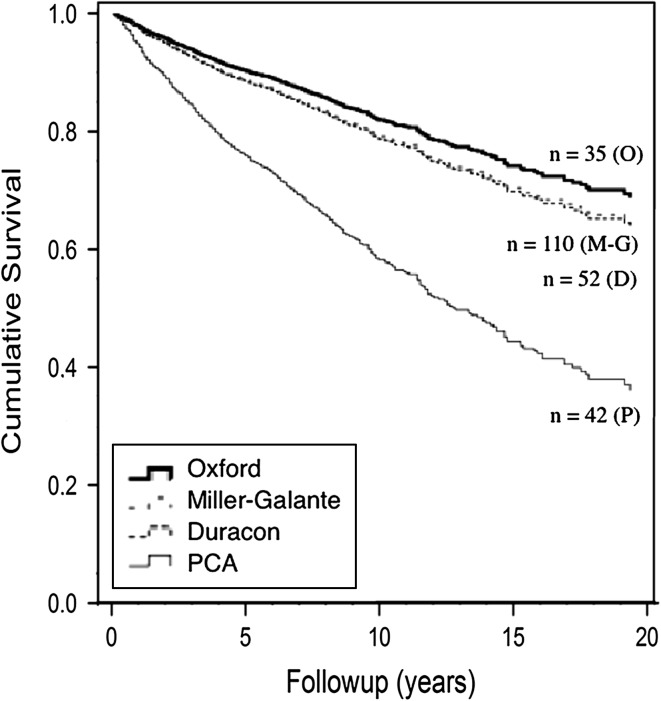
Fig. 5.
The Kaplan-Meier survivorship rates for different UKA designs used during the study period are shown. The end point was defined as any revision, including when either the whole implant or any one component was removed, exchanged, or implanted for any reason. Adjustments were made for age and sex. O = Oxford® (Biomet Inc, Warsaw, IN, USA); M-G = Miller-Galante (Zimmer, Warsaw, IN, USA); D = Duracon™ (Stryker, Mahwah, NJ, USA); P = Porous Coated Anatomic (PCA®) (Howmedica, Rutherford, NJ, USA).