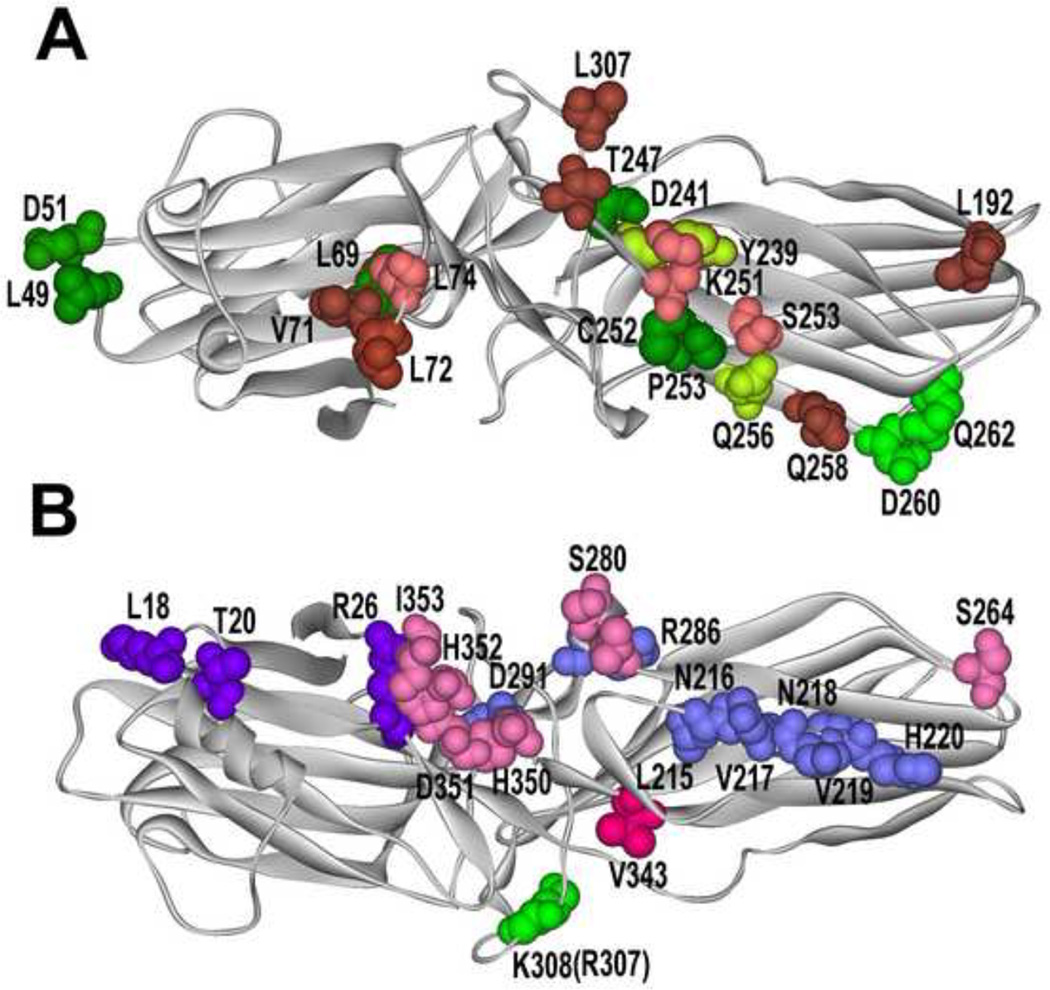
Fig. 1. What arrestin elements do GPCRs and other partners engage?
A. Arrestin from “receptor viewpoint”. Arrestin residues that determine receptor specificity [46,64] are shown in different shades of green: yellow-green, those where mutations (Y239T and Q256Y) result in reduced dopamine D2 receptor binding; bright green, those where mutations (D260K and Q262P) result in high preference for other GPCRs over β2-adrenergic receptor. Elements engaged by Ca2+-liganded calmodulin are shown in pink and brown (darker color indicates greater immobilization by calmodulin [32]). Residues in green with red border (L69 and T239) are shared by receptors and calmodulin. B. Non-receptor- binding side of arrestins. Residues engaged by all forms of PDE4D are shown in blue, those specific for PDE4D5 in bright violet [34], the residue critical cRaf1-binding is in bright green (mutation R307A in arrestin-3 prevents cRaf1 interaction; homologous mutation K308A in arrestin-3 does not) [65], residues responsible for the ability of arrestin-3 to promote JNK3 activation are shown in bright red (critical V343) and pink (supporting residues) [67]. Arrestin-3 structure 3P2D [4] was used to generate this figure (since calmodulin-binding elements were identified in arrestin-2 [32], homologous arrestin-3 residues are highlighted).