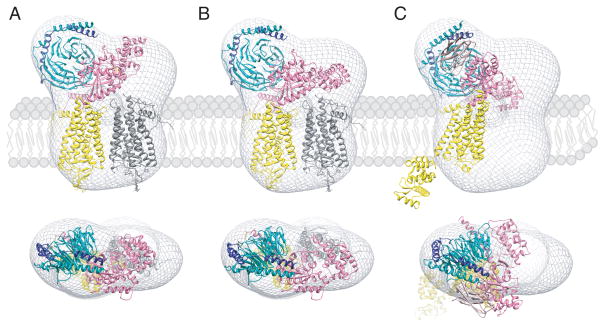
Fig. 4. Orientation of GPCR-G protein complexes in a phospholipid membrane.
Semi-empirical models of the complex formed between light-activated rhodopsin dimer and a Gt heterotrimer (A and B) [52] and the T4L-β2AR-Gs-nanobody complex (C) [48] were fitted into a 3D molecular envelope calculated from projections of negatively stained, bis[succinimidyl] 2,2,4,4-glutarate (DSG) crosslinked rhodopsin*-Gt complexes purified in lauryl maltose neopentyl glycol (LMNG). Fitting of the rhodopsin*-Gt model generated with the structure of inactive Gt (PDB code: 1GOT) leaves a significant unoccupied density above one of the rhodopsin molecules (A), which becomes occupied after a 30o hinge-like motion of the α-helical domain is applied (B). Though fitting the T4L-β2-adrenergic receptor-Gs-nanobody structure into our EM 3D map leaves sufficient space to accommodate a second molecule of this receptor, conformation of the Gsα helical domain is inconsistent with our EM-density (C). Thus, the favored structure of the rhodopsin-Gt complex appears to be that shown in (B). Photoactivated rhodopsin (Rho*) that binds the C-terminal peptide derived from Gtα and the T4L-β2-adrenergic receptor-nanobody molecule are both depicted in yellow. The second rhodopsin molecule in (A) and (B) is shown in gray. Gtα, Gtβ, Gtγ are colored pink, dark cyan and dark blue, respectively.