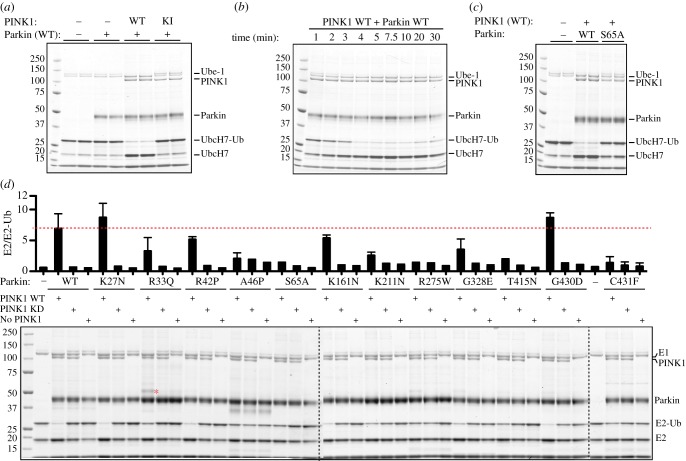
Figure 6.
PINK1-dependent phosphorylation of Parkin Ser65 is required for discharge of ubiquitin from E2. Parkin was phosphorylated using wild-type (WT) or kinase-inactive (KI) MBP-TcPINK1. An E2 discharge assay was established by incubation of this mixture with 2 μg of UbcH7 that had been pre-incubated with 0.5 µg of E1 and FLAG-ubiquitin in the presence of ATP for 60 min. Reactions were allowed to continue for 15 min (a,c,d) or as indicated (b) and stopped using SDS–PAGE loading buffer in absence of reducing agent. Samples were resolved by SDS–PAGE and proteins detected by Colloidal Coomassie staining. (a) Ubiquitin-loaded UbcH7 (UbcH7-Ub) was observed in the absence of Parkin (lanes 1,2). WT Parkin only in the presence of WT MBP-TcPINK1 was able to efficiently discharge UbcH7-Ub (lanes 5,6). No discharge was observed with WT Parkin alone (lanes 3,4) or WT Parkin in the presence of KI MBP-TcPINK1 (lanes 7,8). (b) Time course of E2 discharge after addition of activated WT Parkin in the presence of WT MBP-TcPINK1 demonstrated rapid and maximal discharge of UbcH7-Ub at 4 min. (c) Abrogation of UbcH7-Ub discharge by Parkin Ser65Ala (S65A; lanes 5,6) in contrast to WT Parkin in the presence of WT PINK1 (lanes 3,4). (d) Comparison of the effects Parkin disease mutations on ubiquitin discharge from UbcH7. Red dotted line indicates the WT activity. K27N, R33Q, R42P, K161N, G430D and G328E mutants showed no significant changes in activity. A46P, S65A, K211N, R275W, T415N and C431F displayed markedly decreased E2-ubiquitin discharge ability. Asterisk indicates the R33Q Parkin–ubiquitin thioester. Representative of three independent experiments.