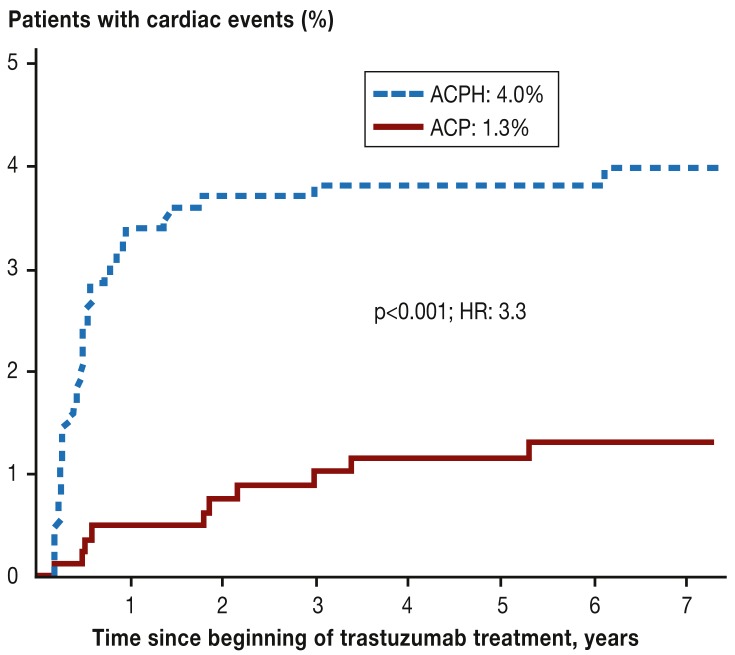
Figure 2.
Cumulative incidence of cardiac events (acute cardiac insufficiency, cardiac death) in the National Surgical Adjuvant Breast and Bowel Project Trial B-31 (NSABP B-31).
ACP: Doxorubicin, cyclophosphamide, paclitaxel; ACPH: Doxorubicin, cyclophosphamide, paclitaxel, trastuzumab; HR, hazard ratio; (according to [24,]: Romond EH et al.: Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel [ACP] with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012; 30: 3792–9. Reproduced with the kind permission of the American Society of Clinical Oncology [ASCO]. The authors, editors, and ASCO are not responsible for errors or omissions in translations)