Figure 8.
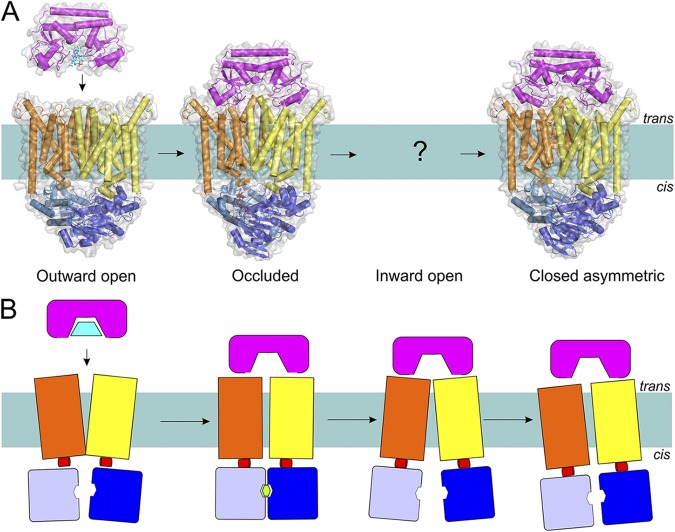
The transport mechanism of Type II importers (exemplified by the BtuC2D2F transporter) based on the available structures (A) and in schematic representation (B). Coloring is as in Fig. 1. Structures have been determined for an outward-open, occluded nucleotide-bound, and closed ATP-free asymmetric transporter (Protein Data Bank accession nos.: 1L7V, 4DBL, and 2QI9; see Table 1 for details of all available structures of ABC transporters). Substrate-loaded BtuF docks onto the resting BtuC2D2 transporter in the outward conformation. ATP binding and concomitant rearrangements in the TMDs lead to the trapping of the substrate between TMDs (substrate was not visible in the crystal structure). The hydrolysis of ATP causes opening of the cytoplasmic gate and allows substrate release, after which the gate is asymmetrically closed, possibly to prevent the leakage of small molecules.