Abstract
A small or sparse population may suffer a reduction in fitness owing to Allee effects. Here, we explored effects of plant density on pollination, reproduction and predation in the alpine herb Pedicularis rex over two years. We did not detect a significant difference in the pollination rate or fecundity (fruit set and the initial seed set) before predation between sparse and dense patches in either year, indicating no pollination-driven Allee effect. However, dense patches experienced significantly fewer attacks by predispersal seed predators in both years, resulting in a significantly decreased realized fecundity (final seed set), suggesting a component Allee effect driven by predispersal seed predation. Predation-driven Allee effects have been predicted by many models and demonstrated for a range of animals, but there is scant evidence for such effects in plants. Our study provides strong evidence of a component Allee effect driven by predation in a plant species.
Keywords: component Allee effect, Pedicularis rex, plant density, predispersal seed predation, reproductive success
1. Introduction
The Allee effect, referring to a positive causal relationship between any component of fitness and population density or size, is of fundamental ecological importance owing to its strong influence on population dynamics ([1–3]; electronic supplementary material, appendix S1). The Allee effect is of particular concern in conservation biology because it may increase the extinction risk of sparse or small populations [3–6]. By contrast, the Allee effect benefits attempts to manage unwanted or alien species because it may limit establishment success or spread of initial colonizers [6–8]. As a process affecting individual fitness, the Allee effect also exerts a selection pressure on the evolution of various life-history traits [6,9].
Many mechanisms can lead to an Allee effect, including mate-finding difficulty, predation, inbreeding depression, reproductive facilitation, interspecific reproductive interference and also other unknown mechanisms [6,10,11]. Species with similar life-history traits may have different responses to population density or size. Thus, predicting Allee effects remains challenging [6,11].
For plant species, the best-documented mechanism of Allee effect is pollination limitation, and it can arise from changes of pollinator composition, reduced pollinator activity, or deceased quantity or quality of pollination service [5,6,10]. Predation is, in theory, another potential mechanism causing an Allee effect, although few empirical studies have demonstrated this possibility in plants [6]. Pedicularis rex is self-compatible but depends exclusively on bumble-bees for reproduction (see electronic supplementary material, appendix S2), and our field survey showed that P. rex suffered severe predispersal seed predation. Therefore, it could potentially experience Allee effects with respect to both pollination success and seed predation. In this study, we explored effects of plant density on pollination, reproduction and seed predation in P. rex over two years in order to test whether Allee effects in reproduction occurred and to investigate potential mechanisms.
2. Material and methods
Fieldwork was conducted during the summers of 2005 and 2011 in Shangri-la in northwestern Yunnan, China. The population studied in 2005 consisted of a sparse patch and a dense patch, occurred on exposed rock outcrops on the south-facing slope of Mt. Wufeng (27°47′46″ N, 99°42′35″ E; ca 3400 m.a.s.l.). The population studied in 2011 occurred in a non-cultivated area of Shangri-la Alpine Botanical Garden where five sparse and six dense patches were sampled (27°54′9-30″ N, 99°38′8-20″ E; electronic supplementary material, appendix S3). There were more than five flowering plants per square metre in dense patches and less than two flowering plants per square metre in sparse patches. We also determined patch size by calculating the number of flowering plants (range: 1–500).
We randomly collected 60 and 175 recently wilted flowers in 2005 and 2011, respectively, and we fixed them in formalin-aceto-alcohol (5% formaldehyde, 5% glacial acetic acid, 50% ethanol) immediately. We then determined the pollination rate (percentage of stigmas on which more than one pollen grain had been deposited) under a fluorescence microscope. To examine reproductive success resulting from natural pollination, we harvested 16 spikes in 2005 (six and 10 spikes from the sparse and dense patches, respectively) and 58 spikes in 2011 (26 and 32 spikes from the sparse and dense patches, respectively) in late August. We counted flowers and fruits produced per plant and seeds per capsule, from which we calculated fruit set per plant and seed set per flower. Predated seeds were easy to distinguish from healthy, intact seeds because the former had lost part of the seed coat. Only intact seeds were included when calculating the final seed set; consequently, seed set was zero when all seeds in the capsule were predated. By contrast, the initial seed set was calculated from both intact and predated seeds per capsule. To quantify fruit and seed predation, we further calculated fruit predation percentage (no. damaged fruits × 100/no. fruits per plant) and seed predation percentage (predated seeds × 100/(intact seeds + predated seeds) per capsule). Seed predation percentage was considered as 100% for fruits in which no distinguishable seeds were found.
We used χ2 independence test to determine differences in the pollination rate between sparse and dense patches in both years, and two sets of analysis of variance (ANOVAs) models to reveal the relationship between patch density and patch means for various measures of reproduction and predation. In the first set, we examined the effects of year, density and their interactions on reproductive success and predation percentage. Plant size and fruit production were also included as a covariate for fruit set and fruit predation, respectively. In the second set, we examined the effects of patch density, size and their interactions on reproductive success and predation in 2011. All statistical analyses were performed with SPSS v. 20.0 (SPSS, Chicago, IL, USA).
3. Results
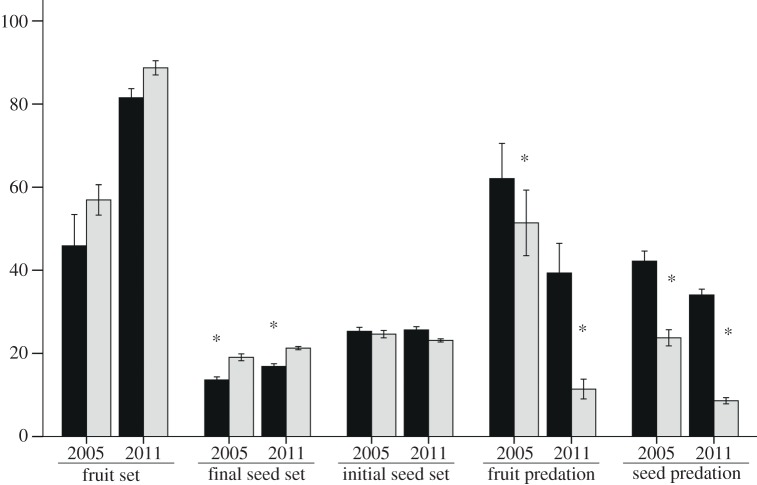
No significant differences were found in the pollination rate between sparse and dense patches in either year (χ2 = 0.29, p = 0.59 in 2005; χ2 = 1.30, p = 0.25 in 2011). Overall, the pollination rate was significantly higher in 2011 than in 2005 (90% versus 63%; χ2 = 22.02, p < 0.001). As a result, fruit set was dramatically higher in 2011 than in 2005. However, there was no significant difference in the initial seed set between years. By contrast, the final seed set was significantly lower in 2005 because P. rex suffered more severe fruit and seed predation that year (table 1 and figure 1).
Table 1.
Analysis of variance to evaluate the differences in reproduction and predation between sparse and dense patches in P. rex, with year, density and their interaction included as fixed effects. Plant size and fruit production were also included as covariates for fruit set and fruit predation, respectively. Asterisks indicate statistical significance of p-values.
| dependent trait | source | d.f. | F |
|---|---|---|---|
| fruit set | density | 1,69 | 2.945 |
| year | 1,69 | 67.671*** | |
| density×year | 1,69 | 0.355 | |
| plant size | 1,69 | 3.855 | |
| initial seed set | density | 1,2598 | 3.767 |
| year | 1,2598 | 0.507 | |
| density×year | 1,2598 | 1.241 | |
| final seed set | density | 1,2926 | 39.025*** |
| year | 1,2926 | 11.954*** | |
| density×year | 1,2926 | 0.426 | |
| fruit predation | density | 1,69 | 6.767* |
| year | 1,69 | 16.580*** | |
| density×year | 1,69 | 0.998 | |
| fruit production | 1,69 | 0.213 | |
| seed predation | density | 1,2926 | 166.220*** |
| year | 1,2926 | 46.571*** | |
| density×year | 1,2926 | 4.250* |
*p < 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Figure 1.
Comparison of reproduction and predation between sparse and dense patches of P. rex in 2005 and 2011. Final seed set was calculated only from the intact seeds, whereas the initial seed set was calculated from both intact and predated seeds. Bars represent s.e. and asterisks (*) represent significant differences (p < 0.05) between sparse (black bars) and dense (grey bars) patches.
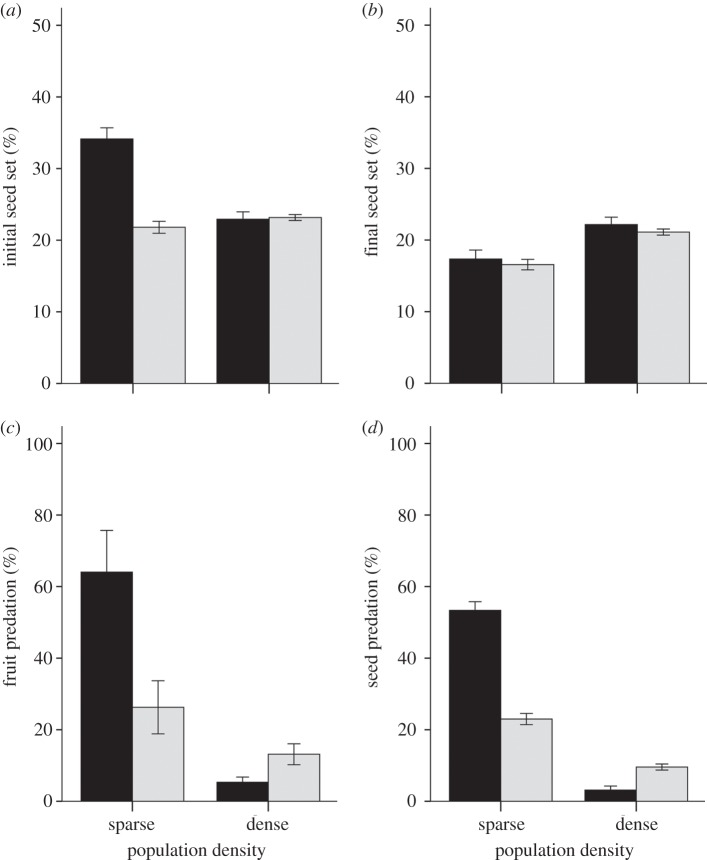
Patch density did not affect fruit set or the initial seed set, but it did significantly influence the final seed set (table 1). The final seed set decreased significantly in sparse patches (figure 1), indicating a component Allee effect. We also found that P. rex experienced lower levels of fruit predation and seed predation in dense patches (table 1 and figure 1). Patch size did not affect fruit set and the final seed set in 2011, but we found a significant interaction between size and density on the initial seed set, fruit and seed predation, respectively (table 2). Initial seed set decreased significantly at high density in small patches but not in large patches (F1,2048 = 59.42, p < 0.001; F1,2048 = 0.38, p = 0.536; respectively), with the highest initial seed set in small sparse patches (figure 2). The percentage of fruit and seed predation decreased at high density more sharply in small patches than in large patches. Moreover, we found distinct patch-size effects on fruit and seed predation between low and high densities (figure 2). Predation percentages were significantly higher in small patches than in large patches at low density (fruit predation: F1,55 = 5.59, p = 0.022; seed predation: F1,2346 = 101.82, p < 0.001; figure 2). By contrast, in high density patches, seed predation was significantly higher in large patches (F1,2346 = 71.92, p < 0.001; figure 2) and a similar trend was seen for fruit predation (F1,55 = 1.39, p = 0.224).
Table 2.
Effects of population density, population size and their interaction (density×size) on reproduction and predation for P. rex patches in 2011 (analysed with two-way ANOVAs). Asterisks indicate statistical significance of p-values.
| dependent trait | source | d.f. | F |
|---|---|---|---|
| fruit set | density | 1,54 | 2.368 |
| size | 1,54 | 0.206 | |
| density×size | 1,54 | 3.060 | |
| initial seed set | density | 1,2047 | 27.348*** |
| size | 1,2047 | 41.221*** | |
| density×size | 1,2047 | 44.556*** | |
| final seed set | density | 1,2345 | 26.958*** |
| size | 1,2345 | 1.025 | |
| density×size | 1,2345 | 0.023 | |
| fruit predation | density | 1,54 | 26.314*** |
| size | 1,54 | 4.573* | |
| density×size | 1,54 | 10.605** | |
| seed predation | density | 1,2345 | 317.878*** |
| size | 1,2345 | 45.029*** | |
| density×size | 1,2345 | 106.270*** |
*p < 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Figure 2.
Average (±s.e.) of (a) the initial seed set, (b) the final seed set, (c) fruit predation and (d) seed predation in small (black bars; less than 20 flowering individuals) and large (grey bars; more than 20 flowering individuals) patches, and in sparse and dense patches of P. rex in 2011.
4. Discussion
Fruit set in P. rex was determined by pollination service, and pollination rate differences arise from differences in pollinator abundance between populations. In 2011, there were more co-flowering plants and larger population sizes at the study site, which increased the overall attractiveness to pollinators both at community and population levels. Both plants and flowers thus received significantly more pollinator visits in 2011 than in 2005 (J Xia, SG Sun & GH Liu 2013, unpublished data). However, the differences in pollinator abundance did not translate into differences in the initial seed set.
Various mechanisms may cause component Allee effects in reproduction, and among these mechanisms, pollination limitation is the most common and well documented for plant species [5,6,12]. Unexpectedly, we did not find significant differences in pollination, fruit set or the initial seed set, indicating that pollination-driven Allee effects did not occur for P. rex. Apart from pollination, predation is another factor affecting reproductive success, which is also density dependent. Therefore, whether plant density has a net positive or negative effect on the realized reproductive success depends on the relative strengths of pollination success and predispersal seed predation [13–17]. For example, decreased predation at low density counterbalanced the disadvantages of low-pollination success, and thus the final output was not detected as density dependent in Acer mono [17]. However, decreased predation at low density was not strong enough to offset decreased fruit set, resulting in an overall net positive effect of density on realized reproduction in Jacaranda copaia [13]. In this study, pollination success and reproduction at the earlier stage were not associated with plant density. However, we did find negative density-dependent predispersal predation in P. rex. As a result, a net positive effect of density on reproduction was detected. The final seed set decreased significantly in the sparse patches both in 2005 and 2011, suggesting that a component Allee effect in P. rex actually was driven by predispersal seed predation. Although predation also decreased as density increased, the Allee effect found in Cistus ladanifer was rather pollination-driven than predation-driven, because decreased predation at high density owing to dilution effect and predator satiation intensified positive density-dependent pollination [16]. Besides plant density, patch size also significantly influenced seed predation percentage in P. rex. However, whether the patch-size effect on predation was negative or positive depended on plant density, with small patches experiencing greater predation percentages at low density but lower levels of predation at high density. This interesting result may be attributable to the different distribution patterns of dense and sparse patches in 2011. Dense patches were distributed much more continuously, and predators might avoid small patches at high density.
In conclusion, a number of modelling studies have shown that a predation-driven Allee effect may occur in theory, and also a wide range of studies on animals have confirmed this mechanism [6,14,18–20]. However, there has been little evidence of predation-driven Allee effects in plants, and our study provides strong evidence of a component Allee effect driven by predispersal seed predation in a plant species.
Acknowledgements
We thank Ben Montgomery for language revision.
Data accessibility
Data were deposited in dryad: doi:10.5061/dryad.6cv06.
Funding statement
This work was supported by the National Natural Science Foundation of China (30900127).
References
- 1.Herrando-Pérez S, Delean S, Brook B, Bradshaw CA. 2012. Density dependence: an ecological Tower of Babel. Oecologia 170, 585–603 (doi:10.1007/s00442-012-2347-3) [DOI] [PubMed] [Google Scholar]
- 2.Sutherland WJ, et al. 2013. Identification of 100 fundamental ecological questions. J. Ecol. 101, 58–67 (doi:10.1111/1365-2745.12025) [Google Scholar]
- 3.Stephens PA, Sutherland WJ. 1999. Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol. Evol. 14, 401–405 (doi:10.1016/S0169-5347(99)01684-5) [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DP, Wittmer HU. 2011. Incorporating Allee effects into reintroduction strategies. Ecol. Res. 26, 687–695 (doi:10.1007/s11284-011-0849-9) [Google Scholar]
- 5.Xia J, Lu J, Wang ZX, Hao BB, Wang HB, Liu GH. 2013. Pollen limitation and Allee effect related to population size and sex ratio in the endangered Ottelia acuminata (Hydrocharitaceae): implications for conservation and reintroduction. Plant Biol. 15, 376–383 (doi:10.1111/j.1438-8677.2012.00653.x) [DOI] [PubMed] [Google Scholar]
- 6.Courchamp F, Berec L, Gascoigne J. 2008. Allee effects in ecology and conervation. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Tobin PC, Berec L, Liebhold AM. 2011. Exploiting Allee effects for managing biological invasions. Ecol. Lett. 14, 615–624 (doi:10.1111/j.1461-0248.2011.01614.x) [DOI] [PubMed] [Google Scholar]
- 8.Boukal D, Berec L. 2009. Modelling mate-finding Allee effects and populations dynamics, with applications in pest control. Popul. Ecol. 51, 445–458 (doi:10.1007/s10144-009-0154-4) [Google Scholar]
- 9.Weber A, Kolb A. 2013. Local plant density, pollination and trait-fitness relationships in a perennial herb. Plant Biol. 15, 335–343 (doi:10.1111/j.1438-8677.2012.00645.x) [DOI] [PubMed] [Google Scholar]
- 10.Gascoigne J, Berec L, Gregory S, Courchamp F. 2009. Dangerously few liaisons: a review of mate-finding Allee effects. Popul. Ecol. 51, 355–372 (doi:10.1007/s10144-009-0146-4) [Google Scholar]
- 11.Kyogoku D, Nishida T. 2012. The presence of heterospecific males causes an Allee effect. Popul. Ecol. 54, 391–395 (doi:10.1007/s10144-012-0313-x) [Google Scholar]
- 12.Johnson SD, Torninger E, Ågren J. 2009. Relationships between population size and pollen fates in a moth-pollinated orchid. Biol. Lett. 5, 282–285 (doi:10.1098/rsbl.2008.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones FA, Comita LS. 2010. Density-dependent pre-dispersal seed predation and fruit set in a tropical tree. Oikos 119, 1841–1847 (doi:10.1111/j.1600-0706.2010.18547.x) [Google Scholar]
- 14.Pavlová V, Berec L, Boukal DS. 2010. Caught between two Allee effects: trade-off between reproduction and predation risk. J. Theor. Biol. 264, 787–798 (doi:10.1016/j.jtbi.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 15.Lutscher F, Iljon T. 2012. Competition, facilitation and the Allee effect. Oikos 122, 621–623 (doi:10.1111/j.1600-0706.2012.20222.x) [Google Scholar]
- 16.Metcalfe D, Kunin W. 2006. The effects of plant density upon pollination success, reproductive effort and fruit parasitism in Cistus ladanifer L. (Cistaceae). Plant Ecol. 185, 41–47 (doi:10.1007/s11258-005-9082-3) [Google Scholar]
- 17.Shibata M, Kikuchi S, Tanaka H, Sueyoshi M, Yoshimaru H, Niiyama K. 2009. Effects of population density, sex morph, and tree size on reproduction in a heterodichogamous maple, Acer mono, in a temperate forest of Japan. Ecol. Res. 24, 1–9 (doi:10.1007/s11284-008-0474-4) [Google Scholar]
- 18.Gascoigne JC, Lipcius RN. 2004. Allee effects driven by predation. J. Appl. Ecol. 41, 801–810 (doi:10.1111/j.0021-8901.2004.00944.x) [Google Scholar]
- 19.McLellan BN, Serrouya R, Wittmer HU, Boutin S. 2010. Predator-mediated Allee effects in multi-prey systems. Ecology 91, 286–292 (doi:10.1890/09-0286.1) [DOI] [PubMed] [Google Scholar]
- 20.Bourbeau-Lemieux A, Festa-Bianchet M, Gaillard J-M, Pelletier F. 2011. Predator-driven component Allee effects in a wild ungulate. Ecol. Lett. 14, 358–363 (doi:10.1111/j.1461-0248.2011.01595.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were deposited in dryad: doi:10.5061/dryad.6cv06.