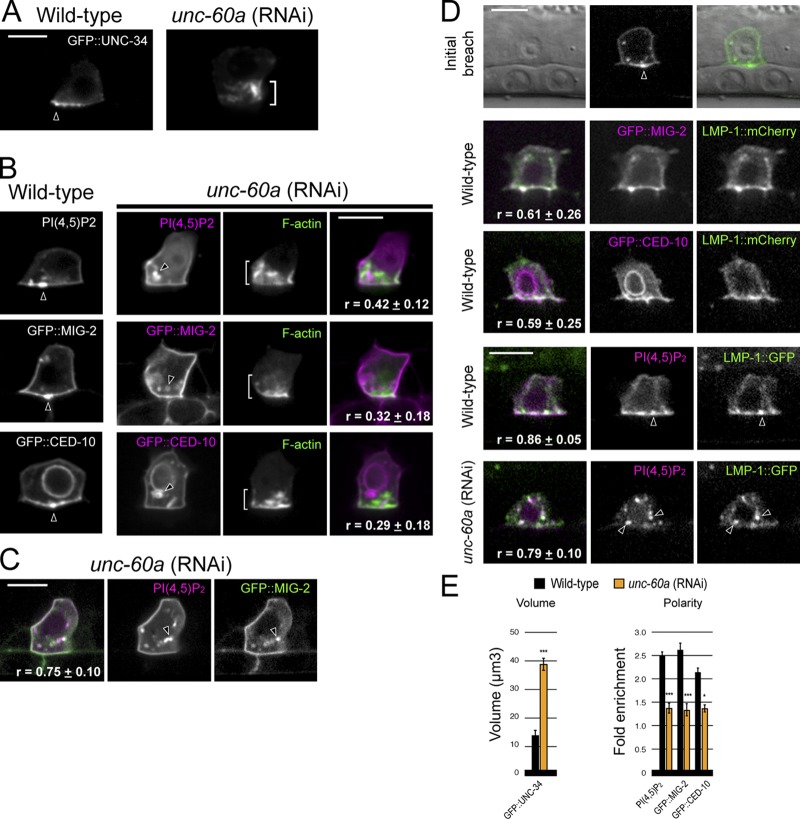
Figure 4.
UNC-60A (ADF/cofilin) regulates the invadopodial membrane. (A) In contrast to its tight localization at the invasive cell membrane in wild-type animals (arrowhead), GFP::UNC-34 (Ena/VASP) localizes to the expanded F-actin network (bracket) after unc-60a RNAi treatment. (B) The invadopodial membrane components PI(4,5)P2 (mCherry::PLCδPH) and the Racs GFP::MIG-2 and GFP::CED-10 were no longer localized to the invasive cell membrane after loss of unc-60a and were found within intracellular vesicles (arrowheads) independent of the expanded F-actin (brackets; r-value reports average Pearson’s correlation coefficient for colocalization within the AC ± SEM). (C) The invadopodial membrane components PI(4,5)P2 and GFP::MIG-2 colocalized within the same vesicles (arrowheads) after loss of unc-60a (r-value reports average Pearson’s correlation coefficient for colocalization within the AC ± SEM). (D) The endolysosome marker LMP-1::GFP localized to invadopodial structures and at the initial breach site (top, arrowhead). LMP-1::GFP colocalized with invadopodial membrane markers (GFP::MIG-2, GFP::CED-10, and PI(4,5)P2) in wild-type ACs (middle panels, arrowheads) and after loss of unc-60a within internalized vesicles (bottom, arrowheads). r-value reports average Pearson’s correlation coefficient for colocalization within the AC ± SEM. (E) Graphs show the volume of GFP::UNC-34 and the polarized localization of the invadopodial membrane components in wild-type (black) and unc-60a (RNAi) animals (orange; n ≥ 6 animals examined for each; *, P < 0.01; ***, P < 0.0001; Student’s t test; error bars indicate ±SEM). Bars, 5 µm.