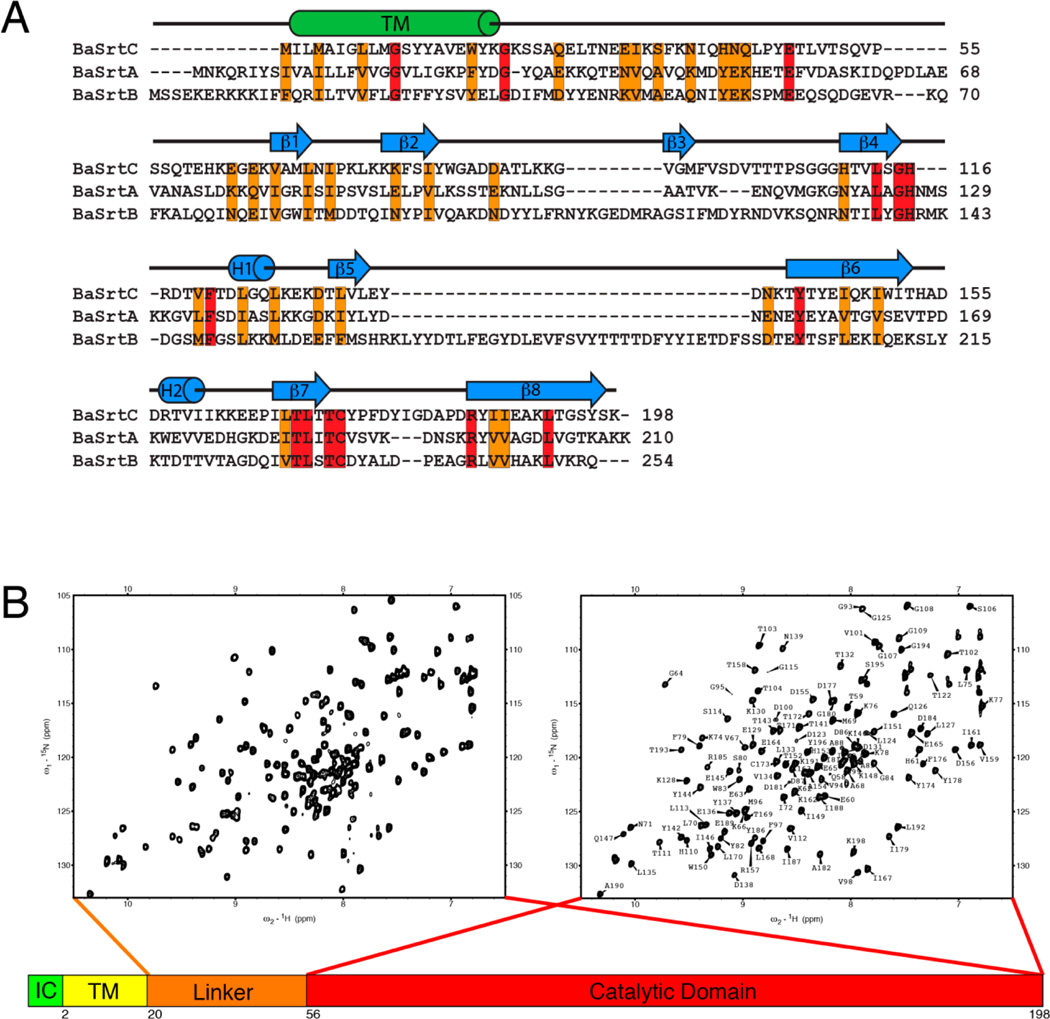
Figure 1.
NMR spectra and amino acid sequence of BaSrtC. (A) Sequence alignment of B. anthracis (Ames strain) class A sortase (BaSrtA), class B sortase (BaSrtB), and class D sortase (BaSrtC). The sequence alignment was performed by ClustalW.63 Conserved residues are colored orange, while identical residues are colored red. The predicted transmembrane region (TM) is indicated by a cylinder labeled TM. Secondary structure features of BaSrtC are indicated by cylinders or arrows above the sequence. (B) 1H–15N HSQC spectra of BaSrtCΔ19 (left) and BaSrtCΔ55 (right). BaSrtCΔ19 contains approximately 30 more peaks, the majority of which reside toward the center of the spectrum. The BaSrtCΔ55 HSQC spectrum has its residue assignments indicated.