Figure 6.
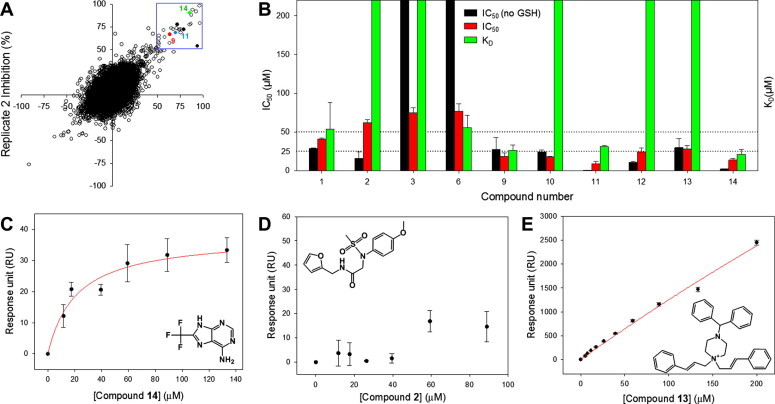
Primary hits from HTS and hit validation using SPR binding assay. (A) Replicate plot from screening 41,022 compounds from structurally diverse in-house, Prestwick FDA-approved drugs, Maybridge and Life Chemicals libraries. The blue box indicates hit compounds with over 50% inhibition at 50 μM compound concentration. The initial percent inhibitions of the three confirmed hits from HTS are shown in red (9), blue (11) and green (14). (B) IC50 values and the dissociation equilibrium constants (KD) of four virtual screening and six HTS hits. All data were normalized for immobilization levels of target and reference proteins. Bars that reach the top of the graph represent KD values of over 200 μM (no binding). (C) The binding data of compound 14 fitted to a single rectangular hyperbolic equation (See Methods). The determined KD of compound 14 was 21.3 ± 6.3 μM, similar to its IC50 value (13.9 ± 2.2 μM). (D) Response units of compound 2 at a series of increasing concentrations (0–90 μM), showing lack of binding to 3CLpro. (E) Response units of compound 13 at a series of increasing concentrations (0–200 μM), showing non-specific binding to 3CLpro.
