Abstract
Marital quality is an important factor for understanding the relationship between marriage and health. Low-quality relationships may not have the same health benefits as high-quality relationships. To understand the association between marital quality and health, we examined associations between two indicators of marital quality (marital support and marital strain) and two biomarkers of inflammation (interleukin-6 and C-reactive protein) among men and women in long-term marriages using data from the Survey of Midlife in the United States (N = 542). Lower levels of spousal support were associated with higher levels of inflammation among women but not men. Higher levels of spousal strain were weakly and inconsistently associated with higher levels of inflammation among women and men; the effects were diminished with the addition of psychosocial and behavioral covariates. These findings suggest marital quality is an important predictor of inflammation, especially among women.
Keywords: gender, health, midlife, satisfaction, sociobiology
Social relationships play a significant role in health outcomes ranging from catching a cold (Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997) to mortality (Holt-Lunstad, Smith, & Layton, 2010). A marital relationship is one of the most important social relationships established in adulthood and the better health experienced by married adults is well documented (Waite & Gallagher, 2000). Married adults live longer (Johnson, Backlund, Sorlie, & Loveless, 2000), rate their health better (Williams & Umberson, 2004), and report fewer chronic conditions and functional limitations (Hughes & Waite, 2009) compared to their nonmarried counterparts. While prior research has established that marriage confers health benefits, there has been less focus on how the health of married individuals may vary as a function of the quality of a marriage and the duration of time spent in a marriage. Larger studies have generally focused on the association between marital quality and broadly defined health outcomes, whereas smaller laboratory-based studies have focused on experimentally induced marital conflict or collaboration and physiological processes (Carr & Springer, 2010). Thus it is important to examine the association between marital quality and physiological processes related to health in a population-based study of adults. Greater understanding of physiological processes among individuals in long-term marriages could enhance our understanding of the health benefits associated with long, high-quality marriages and the health risks associated with long, low-quality marriages.
The present study aims to evaluate one hypothesized physiological pathway through which marriage may influence health by linking the quality of marital relationships to two biomarkers indicating inflammatory processes: Interleukin-6 (IL-6), a proinflammatory cytokine, and C-reactive protein (CRP), an acute phase protein. Social relationships have shown strong associations with many diseases also associated with inflammatory processes, such as cardiovascular disease and cancer, indicating that inflammation may be an important pathway linking the social environment to health outcomes (Kiecolt-Glaser, Gouin, & Hantsoo, 2010). It is hypothesized that psychological stress activates a cascade of physiological processes resulting in the production of proinflammatory cytokines (e.g., IL-6, CRP), contributing to reductions in cell division and accelerated cellular aging (Epel, 2009). Marital relationships may provide social and emotional support that can buffer the effects of other life stressors, leading to decreased inflammation and better health outcomes (Cohen & Wills, 1985; Ross, 1995). Conversely, marriage may be a source of stress in its own right and the strain associated with marital conflict, increased demands and obligations, or both may lead to increased inflammation and subsequently poorer health outcomes (Berkman, Glass, Brissette, & Seeman, 2000). Thus, inflammation may be one key pathway linking the quality of a marital relationship to health outcomes.
Several theoretical frameworks have been important to understanding how marital relationships influence health. For the purpose of the current study, we predominantly draw from social relationships theories, examining marriage as a social relationship that may confer both social support and social stress. Social relationships theories (i.e., stress-buffering and social integration) posit that marriage is a source of social support (emotional, tangible, informational) that buffers the effects of life stress (Cohen & Wills, 1985; Ross, 1995), and that marriage can be a form of social integration that encourages salutary behaviors through social control and results in positive psychological characteristics (e.g., self-esteem, identity) that increase health (House, Landis, & Umberson, 1988; Seeman, 1996).
Marital status and marital history (i.e., number of divorces or widowhoods, length of time spent in marriage, age at first marriage) have been related to increased mortality (Dupre, Beck, & Meadows, 2009; Molloy, Stamatakis, Randall, & Hamer, 2009), chronic conditions, decreased functional status, and self-rated health (Dupre & Meadows, 2007; Hughes & Waite, 2009). Although research on marital history considers a variety of factors, much of this research suggests that increased marital stability (i.e., greater duration) is linked to better health. If the duration of marriage is an important factor in predicting health, it is likely contextualized by the quality of the marriage. Marital quality is related to couples remaining married (Gottman & Levenson, 1992) and is also likely to be important to understanding how marriage affects health over time. Thus, long, supportive marriages should be salutary for one's health, and long, conflict-ridden marriages should be pernicious.
Marital quality is important for understanding how marriage influences health (Kiecolt-Glaser & Newton, 2001). Historically, research on marital quality has focused on a single dimension of marital quality—marital satisfaction. Marital satisfaction provides a global assessment of one's marriage, but it disregards the multidimensionality of the marital relationship (Fincham & Linfield, 1997). Examining positive and negative evaluations of a marital relationship can provide more nuanced information regarding various dimensions of marital quality and how they are differentially associated with health.
As a form of social support, a marital relationship is thought to reduce the appraisal of an event as being stressful, promote positive coping strategies, and reduce negative emotional responses to stress (Cohen & Wills, 1985). An experiment examining neurological responses to physical stress and pain among women found that wives subjected to a shock while alone had greater neurological stress responses than wives subjected to the same stress while holding their husbands’ hand. Furthermore, these stress responses varied as a function of marital satisfaction, with less response observed among individuals reporting higher levels of satisfaction (Coan, Schaefer, & Davidson, 2006). However, a marital relationship does not always provide social support, nor does it guarantee that the support provided by one spouse will meet the needs of the other spouse (Uchino, Vaughn, & Matwin, 2008). If a marital relationship is not a source of support, it may not reduce the impact of other life stressors; individuals in marital relationships that are unsupportive or are not a source of stability or satisfaction may not experience the expected health benefits of marriage. Thus, we expect that the presence of a supportive spouse will reduce neurological responses to stress, therefore reducing the subsequent cascade of inflammatory cytokines and acute phase proteins, such as IL-6 and CRP.
On the other hand, if relationships are unsupportive or are a source of strain and conflict, they may be independent sources of stress, and may exacerbate the effects of other life stressors on health (Seeman, 1996; Uchino, 2009). Conflict, disagreement, and hostility between marital partners have shown deleterious effects on the cardiovascular, neuroendocrine, and immune systems in laboratory experiments (Robles & Kiecolt-Glaser, 2003; Smith et al., 2009). In laboratory settings, marital conflict has also been associated with increases in IL-6; these increases were not observed when couples participated in the control task and did not engage in hostile behaviors (Gouin et al., 2009). Taken together, these studies indicate that in laboratory settings where conflict is induced, married couples experience physiological stress responses. One implication of such findings is that, if these responses are repeated over many years outside the lab, they may have long-term health implications (Manuck, 1994; McEwen & Seeman, 1999). Thus, we expect that over time, couples with high conflict, disagreement, and hostility will have higher levels of inflammation and greater levels of cardiovascular disease risk because they engage in these behaviors frequently.
Assessing marital quality is important to understanding heterogeneity in the health of the married. Examining both positive and negative aspects of marital quality (e.g., satisfaction, support, strain) can provide a more detailed examination of how the various facets of marital quality may differentially predict downstream heath outcomes.
Inflammation, Health, and Marriage
Inflammation plays a substantial role in aging and is considered to be a key process linking environmental stressors to longevity and age-related diseases such as cardiovascular disease, diabetes, and cancer (Crimmins & Finch, 2006; Finch, 2011). IL-6 and CRP are two indicators of general systemic inflammation that can provide information regarding the links between social relationships and disease etiology (Kiecolt-Glaser et al., 2010). IL-6 and CRP are part of both the adaptive and innate immune systems (Finch, 2011). IL-6 is an inflammatory cytokine that stimulates the production of several acute phase proteins, including CRP, which is created in the liver and is responsible for reduced endothelial vasodilation and increased platelet aggregation (Kishimoto, 2005). IL-6 is expressed in many cells, including macrophages and adipose (fat) cells, with higher levels of expression in visceral fat cells, or the abdominal fat cells that surround internal organs. IL-6 and CRP are seen as both causes and consequences of health change with aging and have been associated with cardiovascular disease (Danesh et al., 2008), diabetes (Festa, D’Agostino, Tracy, Haffner, & Insulin Resistance Atherosclerosis Study, 2002), cognitive decline (Gimeno et al., 2009), disability (Ferrucci et al., 2005), and mortality (Alley, Crimmins, Bandeen-Roche, Guralnik, & Ferrucci, 2007).
Basal levels of IL-6 and CRP are good proximal markers of disease risk and may become dysregulated with chronic exposure to social stress (Alley et al., 2007). IL-6 and CRP are always present in the body at low levels and can become rapidly elevated by acute illness and psychological stress, returning to normal basal levels shortly after recovery from infection or stress. Research indicates that chronic stressors such as poverty and caregiving, and chronic health conditions such as obesity and cardiovascular disease, are related to elevated basal levels of IL-6 and CRP (Alley et al., 2006; Kiecolt-Glaser, Bane, Glaser, & Malarkey, 2003). Thus, inflammation is a plausible physiological pathway linking marriage to health. It has been shown to be associated with marital quality in laboratory studies (Gouin et al., 2009), and therefore it makes sense to study the association between marital quality and inflammation in a population-based study of individuals in long-term marriages.
Marriage may confer health differently for men and women. Men and women differ in their social networks and their sensitivity to relationship characteristics (Baumeister & Sommer, 1997). Therefore, different facets of marital quality may differentially confer health benefits or costs among men and women. Men have larger social networks and are more inclusive of others, whereas women tend to have smaller social networks focused on developing stronger bonds among relatively few social ties (Baumeister & Sommer). Thus, men may experience health benefits from the social tie associated with marriage, as evidenced by previous research indicating that marriage is more beneficial for men than for women, but women may be more sensitive to the quality of their marital relationship. Women tend to become more emotionally upset than men during disagreements (Almeida & Kessler, 1998) and are more sensitive to threats associated with the disruption of relationship harmony, whereas men tend to be more sensitive to threats associated with loss of dominance, power, and control (Smith, Gallo, Goble, Ngu, & Stark, 1998). Thus, women may be more sensitive to the absence of positive qualities, such as support, affection, and satisfaction, compared to men; men may be more sensitive to changes in marital status and marital strain or conflict over issues of power and control. To better understand how marital quality influences inflammation in men and women, we must examine both positive and negative marital characteristics, which may aid in our understanding of various mechanisms leading to gender differences in associations between marriage and inflammation (Kiecolt-Glaser & Newton, 2001).
Gender differences in immune response have been found among couples participating in laboratory conflict tasks, but the evidence is contradictory. Individuals that report more negative behaviors during arguments in general, as well as those engaging in hostile and negative behaviors in laboratory conflict resolution tasks, were found to have reduced blastogenic responses to mitogens, reduced NK cell lysis, and increased Epstein–Barr virus antibody titers, compared to those reporting or engaging in fewer of these behaviors—and these effects were greater among women than men (Kiecolt-Glaser et al., 2005). Other laboratory studies have shown acute increases in IL-6 among individuals who display negative behaviors while participating in a conflict task and among couples displaying hostile behaviors during marital interactions, but have not found gender differences in these associations (Gouin et al., 2009). These conflicting results may be due to several reasons: First, individuals engaging in hostile behaviors during induced laboratory conflict engage in the conflict task regardless of whether they would normally engage in conflict. Second, previous research has indicated that a substantial percentage of married participants refuse to participate in laboratory studies of marital conflict after learning that a study includes discussion about a current marital problem (Miller, Dopp, Myers, Stevens, & Fahey, 1999). Thus, using a population survey may better capture chronic conflict and feelings of support, and may have better participation rates for all couples. These methodological differences may increase the ability to detect gender differences in the association between marital quality and health.
One recent study using a subset of the Midlife in the United States (MIDUS) cohort, a longitudinal study of health and well-being of Americans aged 35–86, examined gender differences in the association between marital quality and IL-6 and found that marital satisfaction and marital strain were associated with increased IL-6 for women younger than 53 but not for older women or for men (Whisman & Sbarra, 2012). The current study differs from the Whisman and Sbarra study with respect to the sample used in the analyses (the current sample, even when restricted to those married for 10 or more years, is larger) and because the current study accounts for marital duration. The latter may be important to understanding the role of marital quality and inflammatory processes. Examining shorter marriages may result in examining younger couples and less stable marriages, where influences on IL-6 and CRP could be a result of factors that are also related to marital quality such as a previous but recent marriage and age.
The aim of the current study is to examine links between marital quality—defined by marital support and marital strain—and inflammatory processes as one potential pathway by which marriage may influence health over the life cycle. To understand the effects of marital quality among couples in long-term relationships, we examine only individuals married to the same person for 10 or more years. Restricting the analyses to individuals married to the same person for 10 or more years also has the benefit of eliminating individuals in short marriages, where inflammatory loads may be more indicative of other factors that may confound associations between marital quality and inflammation (e.g., previous marriage, age). We examine associations between positive and negative marital characteristics—support and strain—and IL-6 and CRP, hypothesizing that higher levels of marital strain and lower levels of marital support will be associated with higher levels of inflammatory markers IL-6 and CRP. We also examine gender differences in these associations, hypothesizing that women's inflammatory levels will be more strongly associated with marital support and strain compared to men's, and that, for men, inflammatory levels will be associated with marital strain and conflict, but not support, due to men's greater sensitivity to threats to dominance and control.
Several demographic, behavioral, and psychosocial factors are established corollaries of both inflammation and social relationships. Age is associated with higher levels of IL-6 and CRP and is associated with many diseases of aging (Crimmins, Vasunilashorn, Kim, & Alley, 2008). Socioeconomic status gradients in IL-6 and CRP have been observed among British civil servants (Gimeno et al., 2009) and in U.S. populations (Friedman & Herd, 2010), although some studies have only observed social differences at very high levels of CRP (Alley et al., 2006).
Behavioral factors have also been associated with inflammation and social relationships. Smoking has been consistently associated with higher levels of CRP and IL-6 (Hamer & Chida, 2009). Regular physical activity and exercise have been associated with lower levels of IL-6 and CRP in population studies (Colbert et al., 2004; Singh & Newman, 2010). Obesity—particularly abdominal obesity—has been associated with increased inflammation, due to the release of IL-6 by visceral (abdominal) adipose tissue cells in close proximity to the liver, which causes greater production of CRP (Fontana, Eagon, Trujillo, Scherer, & Klein, 2007). Abdominal adiposity has also been related to higher levels of social stress in adults (Brunner, Chandola, & Marmot, 2007) and children (Donoho, Weigensberg, Emken, Hsu, & Spruijt-Metz, 2010). The use of statin medications, prescribed for lowering cholesterol, has been associated with lower levels of inflammation, including CRP and IL-6 (März, Winkler, Nauck, Böhm, & Winkelmann, 2003). Poor sleep quality has been associated with higher levels of CRP (Matthews et al., 2010) and has also been associated with marital quality (Friedman et al., 2005). Lastly, depression has shown consistent associations with elevated IL-6 and CRP (Howren, Lamkin, & Suls, 2009) and has also been associated with higher levels of marital conflict (Choi & Marks, 2008) and lower levels of marital satisfaction (Renshaw, Blais, & Smith, 2010). Thus, it is crucial to examine the effects of these potential confounders of the association between marital quality and inflammation.
Method
We analyzed data from the Survey of Midlife in the United States (MIDUS) Biomarker Study. The MIDUS study was initially conducted by the MacArthur Foundation Research Network on Successful Midlife Development in 1995–1996 (MIDUS 1). The original sample was a national probability sample of noninstitutionalized, English-speaking midlife adults (age range 25–74) residing in the 48 contiguous states, with an additional sample of twins/triplets identified from an ongoing database of twins. An average of 9.2 years after completing MIDUS 1 (range = 7.8 to 10.4 years), participants were asked to participate in a telephone interview and subsequent postal survey (MIDUS 2) similar in content to MIDUS 1. Participants who completed the MIDUS 2 phone interview were subsequently asked to participate in the Biomarker Study. The Biomarker Study involved an overnight hospital stay at one of the three general clinical research centers in the United States (University of Wisconsin–Madison; University of California, Los Angeles; Georgetown University) and included a detailed health interview, a physical health examination, the collection of biological specimens, and an additional psychosocial questionnaire to assess various physiological states. Participants completed the biomarker study an average of 1.91 years after completing the MIDUS 2 phone interview. The age range of the sample at the time of the biomarker collection was 35 to 86. A detailed description of the sampling and participation rates across the MIDUS studies is provided elsewhere (Dienberg Love, Seeman, Weinstein, & Ryff, 2010).
The analytic sample was restricted to individuals who were married to the same person at the time of the biomarker study and at MIDUS 1. This sample allowed for the examination of health differences associated with marital quality for individuals in long-term relationships. We also restricted our analyses to non-Hispanic White race/ethnicity because of the small number of sample members with other ethnicities: Hispanic White (n = 6), Native American (n = 3), African American (n = 12), Multiracial/Other (n = 11). This resulted in an analytic sample of 553 participants (264 women), including 70 monozygotic twins, 55 dizygotic twins (1 set of triplets), and 2 siblings. The MIDUS study did not include any spousal dyads, and therefore there are no individuals reporting on the same marriage in this study. Descriptive characteristics of the final analytic sample are presented in Table 1.
Measures
Marital quality
Marital quality was measured with two scales indicating spousal support and spousal strain (Schuster, Kessler, & Aseltine, 1990; Turner, Frankel, & Levin, 1983). Support (α = .90) was assessed using the mean of six items rated on a 4-point Likert scale from 1 = not at all to 4 = a lot. Questions included feelings of being cared for, understood, and appreciated, as well as being able to rely on, open up to, and relax around one's spouse. Strain (α = .87) was assessed using the mean of six items tapping perceptions of criticism, demands, tension, feeling let down, irritability, and arguments, using a 4-point Likert scale from 1 = not at all to 4 = a lot. Marital support and strain scores from MIDUS 2 were used.
IL-6 and CRP
After overnight fasting, blood samples were obtained from all participants in the morning following a standard study protocol (Ryff, Seeman, & Weinstein, 2010). Samples were frozen at -60°C to -80°C and shipped on dry ice to the MIDUS Biocore Laboratory where they were stored at -65°C for monthly batch analysis to ensure consistency across the three collection sites. IL-6 was assayed using Quantikine® High-sensitivity ELISA kits (R&D Systems, Minneapolis, MN). The assay sensitivity, or minimum amount of IL-6 that could accurately be measured using this assay, was 0.16 pg/ml. The inter-assay coefficient of variation (CV), or variation in the control substrate run on each plate used to measure plate-to-plate consistency, was 13%. The intra-assay CV, or variation observed when many duplicate samples are run, was 4.1%. CRP was assayed using particle-enhanced immunonephelometry (BN II nephelometer, Dade Behring, Inc., Deerfield, IL). The assay sensitivity was 0.18 ug/ml, inter-assay CV was 5.7%, and intra-assay CV was 4.4%, varying slightly between the different batches run. The intra- and inter-assay CVs for these samples were well below 20%, an established acceptable range (DeSilva et al., 2003).
Anthropometric, behavioral, and psychosocial variables
Waist and hip circumferences were measured to the nearest millimeter by a trained clinician using a Gulick II tape measure. Waist circumference was measured at the narrowest point between the hips and the iliac crest. Hip circumference was measured at the iliac crest. Age was calculated from self-reported date of birth. Exercise was coded as a dummy variable, with 1 indicating 20 minutes of light, moderate, or vigorous activity three times per week or more and 0 indicating fewer than three times per week. Current smoking status was self-reported and coded as a dummy variable (0 = nonsmoker, 1 = smoker). Sleep quality was measured using the subjective sleep item from the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), “During the past month, how would you rate your sleep quality overall?” Response options included: very good, fairly good, fairly bad, and very bad.
Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES–D, Radloff, 1977). This scale is well validated and has been widely used to characterize depressive symptomatology in large epidemiological studies. The scale contains 20 items tapping feelings of depression (e.g., sad, lonely) absence of positive affect (e.g., happy, joyful), somatic symptoms (e.g., restless, poor appetite), and interpersonal difficulties (e.g., feeling disliked, people were unfriendly) over the past week. Items were rated on a 4-point Likert scale ranging from 1 = rarely or none of the time to 4 = most or all of the time, with higher scores indicating greater symptoms and the mean of the 20 items used. Internal consistency of the scale was high in this sample (α = .89).
Data Analysis
To contextualize the effect of each marital quality characteristic (support, strain), the two indices were examined separately with serum IL-6 and CRP. We used OLS regression with clustered standard errors to account for the nonindependence of the twin and sibling pairs. The initial distributions of IL-6 and CRP were positively skewed; therefore, natural-log transformations were used to normalize these distributions and eliminate issues of heteroscedasticity in multivariate models. Linear models with log-transformed dependent variables yield estimates that, when multiplied by 100, are interpreted as percent change in the dependent variable with a one-unit increase in the independent variable, when all other covariates are held constant. We used a Type 1 error rate of .10 to indicate statistical significance and placed a stronger emphasis on the effect size estimates and their confidence intervals. We interpreted the results using a traditional Type 1 error rate of less than .05 to indicate significance, and interpreted rates between .05 and .10 as being marginally significant. We used a hierarchical modeling strategy (Aiken & West, 1991; Cohen, Cohen, West, & Aiken, 2003), where Model 1 examined the association of each marital quality indicator (support, strain) with each inflammatory marker (Il-6, CRP), controlling for age, gender, and education. In Model 2, the interaction of the quality indicator with gender was added to examine whether the interactions account for greater variance than gender and marital quality alone. In Model 3, smoking, exercise, waist–hip ratio, statin use, sleep quality, and depressive symptoms were added as behavioral and psychosocial covariates that may confound associations between marital quality and inflammation. The interaction was only retained in Model 3 if significant (p < .10) in Model 2. All interaction terms were created with centered, continuous independent variables. When significant, interactions were probed using simple slope analysis (Aiken & West, 1991). Unstandardized coefficients are presented because standardized coefficients are inappropriate in models containing interactions with centered predictors (Aiken & West). All statistical analyses were performed using Stata 11.1 (StataCorp., College Station, TX).
Results
Marital support was negatively correlated with marital strain (r = -.63), and IL-6 was positively associated with CRP (r = .53). Descriptive characteristics of the sample are presented in Table 1. There were significant gender differences in levels of marital support, t(540) = 3.24, p < .001, with men reporting greater levels of support. There were marginal gender differences in marital strain, t(540) = 1.56, p = .06, with men reporting lower levels of marital strain compared to women.
Marital Support and Inflammatory Markers
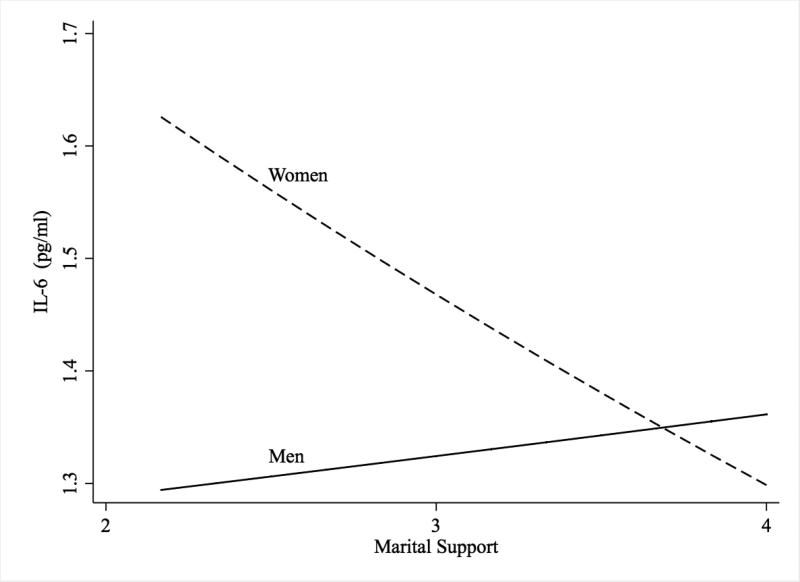
Table 2 presents the results of regression analyses of IL-6 and CRP on support. In the main effects model including age and education (Model 1), support was marginally and negatively associated with IL-6 (b = -0.06, 95% CIs [-0.11, 0.001], p = .06). In Model 2, the addition of the interaction of support and gender was significant (b = -0.15, 95% CIs [-0.27, -0.03], p = .01). The interaction was probed using methods described by Aiken and West (1991), and is shown in Figure 1. Tests of simple slopes indicate that higher support was associated with lower IL-6 among women (b = -0.12, 95% CIs [-0.20, -0.05], p = .001), but not men (b = 0.03, 95% CIs [-0.06, .12], p = .53). This interaction is illustrated in Figure 1. Education was not associated with IL-6, but age was significantly associated with higher IL-6 (b = 0.01, 95% CIs [0.00, 0.01], p < .001). In Model 3, the addition of behavioral covariates did not attenuate the interaction between gender and support. Exercise and the use of statin medications were associated with lower IL-6 (b = -0.08, 95% CIs [-0.14,-0.01], p = .02, and b = -0.06, 95% CIs [-0.12, 0.00], p = .06, respectively), although statin drugs were only marginally associated with IL-6. Waist-to-hip ratio was associated with higher IL-6 (b = 0.77, 95% CIs [0.41, 1.13], p < .001), and depressive symptoms were marginally associated with higher IL-6 (b = 0.004, 95% CIs [0.00, 0.008], p = .08). Sleep and smoking were not significantly associated with IL-6.
Figure 1.
Marital Support and IL-6 for Men and Women.
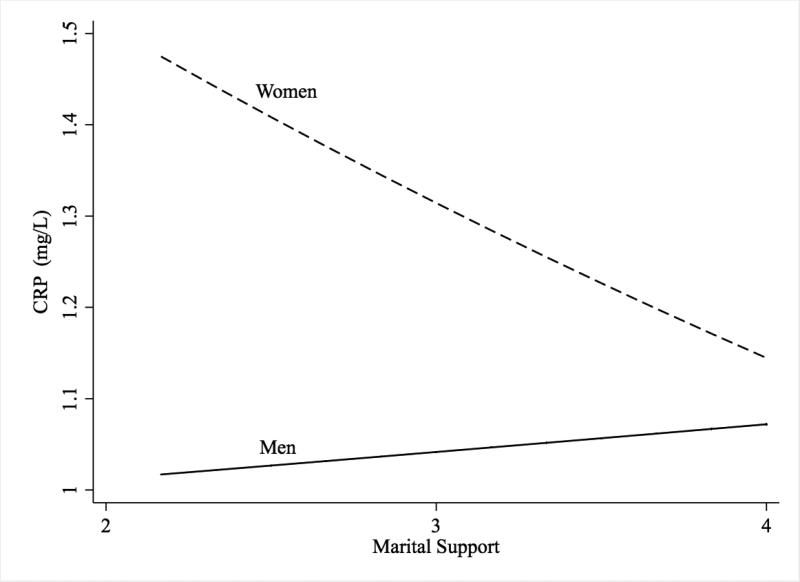
The results of regression analyses of CRP on support are presented in Table 2. In the main effects model including age and education (Model 1), support was not significantly associated with CRP (b = -0.06, 95% CIs [-0.16, 0.03], p = .17). In Model 2, the addition of the interaction of support and gender was marginally significant (b = -0.17, 95% CIs [-0.35, 0.01], p = .07). The interaction was probed using methods described by Aiken and West (1991); tests of simple slopes indicated higher support was associated with lower CRP for women (b = -0.14, 95% CIs [-0.27, -0.02], p = .03), but not for men (b = 0.03, 95% CIs [-0.10, 0.16], p = .42). This interaction is illustrated in Figure 2. Education was not significantly associated with CRP, but age was associated with higher CRP (b = 0.01, 95% CIs [0.00, 0.01], p = .003). In Model 3, the addition of behavioral covariates did not attenuate the interaction between gender and support. Exercise was associated with lower CRP (b = -0.17, 95% CIs [-0.27, -0.07], p = .001), and waist-to-hip ratio was associated with higher CRP (b =1.44, 95% CIs [0.68, 2.21], p < .001). Other behavioral covariates, smoking, use of statin drugs, and depressive symptoms were not significantly associated with CRP.
Figure 2.
Marital Support and CRP for Men and Women.
Marital Strain and Inflammatory Markers
Table 3 presents the regression of IL-6 on marital strain. Strain was associated with higher IL-6 in the main effects model (b = 0.05, 95% CIs [0.004, 0.10], p = .03) controlling for age and gender. The interaction of gender and support was not significant (b = 0.06, 95% CIs [-0.03, 0.16], p = .20) and was omitted from Model 3. The addition of behavioral covariates in Model 3 attenuated the association between strain and IL-6 (b = 0.03, 95% CIs [-0.02, 0.08], p = .19). Marital strain was not associated with CRP in main effects models or in models including interactions with gender (Table 3).
Discussion
Prior research has indicated that being married is salutary for health outcomes (Johnson et al., 2000; Kaplan & Kronick, 2006; Molloy et al., 2009), but additional studies of women have shown marriage to be beneficial only to the happily married (Gallo et al., 2003; Gallo, Troxel, Matthews, & Kuller, 2003). Findings from the current study provide further evidence consistent with the latter point, but provide more nuanced measures of marital quality showing that for women, levels of inflammation are associated with levels of spousal support and are weakly associated with levels of spousal strain; and for men, levels of inflammation are only weakly associated with marital strain. These findings illustrate that differences in marital quality, broadly defined, are important for inflammation among women but may not be as important for men. It also answers important questions concerning two aspects of marital quality—marital support and marital strain—and their associations with two physiological markers of inflammation, IL-6 and CRP (Kiecolt-Glaser & Newton, 2001).
In the current study, marital support was associated with lower levels of CRP and IL-6 among women, but not men. This finding is consistent with prior research indicating that long-term health trajectories are influenced by marital satisfaction among women (Gallo et al., 2003; Gallo, Troxel, Matthews, & Kuller, 2003; Troxel, Matthews, Gallo, & Kuller, 2005). Although we did not examine broader social ties among men, previous research has found that marital status is associated with lower CRP among men but not women (Sbarra, 2009) and that higher levels of marital strain are associated with higher IL-6 among women (Whisman & Sbarra, 2012). In addition, broader measures of social support and integration have typically been related to reductions in CRP among men, but not women. For example, general social support was related to lower CRP in adult men (Mezuk, Diez Roux, & Seeman, 2010) and having a greater number of social ties was related to lower CRP for men, but not for women (Ford, Loucks, & Berkman, 2006). Although the current study does not examine marital status directly, combined evidence from the current study and other studies indicates that men may experience better health, or lower inflammation, in any marriage, while women may only experience better health in a good marriage. This could be because unmarried men engage in unhealthy, higher risk behaviors compared to women. For example, one study found that being unmarried after an unhappy marriage ended resulted in higher alcohol consumption for men, but not for women (Waite, Luo, & Lewin, 2009). Future research should attempt to disentangle gender differences in the roles that marital status and marital quality play in predicting inflammation and other health indicators.
Marital strain was associated with higher levels of IL-6, but not CRP, among both men and women, but this effect was no longer significant in fully adjusted models. This finding indicates that strain is not as strongly or consistently associated with inflammation as is support, and that associations between marital strain and IL-6 may be accounted for by other factors such as obesity, medication use, lack of physical activity, and depressive symptoms. Further studies with longitudinal measures of IL-6 and CRP could examine the potential mediating effects of these behavioral and psychosocial variables in the association between marital strain and inflammation.
We hypothesized that men would be sensitive to issues of dominance, power, and control (Smith et al., 1998), and thus their levels of inflammation would be more sensitive to marital strain than marital support. Our findings provide some evidence indicating that men are more sensitive to marital strain than marital support. Among men, marital support was not associated with IL-6 or CRP, whereas marital strain was associated with higher levels of IL-6 but was not associated with CRP. Our findings do not support our hypothesis that men are more sensitive to strain than women, as there was no interaction between gender and strain in predicting levels of inflammation. These findings are somewhat different than the stronger findings from laboratory studies, where conflict has consistently been associated with acute increases in inflammatory markers, including IL-6 (Kiecolt-Glaser et al., 2005). This could be due to methodological differences, as the participants in laboratory studies are engaging in a conflict task, whereas in this study, participants were reporting on general levels of marital strain. These differences could also be due to the sample, as the individuals in this study were considerably older and were in long-term marriages.
The current study was strengthened by the use of a large sample of U.S. residents, well-validated indicators of immune function, and multiple measures of marital quality, capturing both positive and negative dimensions of marriage. But there are also several notable limitations. First, the study population comprised Caucasian adults married for ten or more years, with relatively high educational attainment. This limits the generalizability of these findings to the broader population of U.S. adults. There is considerable evidence indicating that marriage may be different for individuals of various ethnic, racial, and socioeconomic backgrounds (McLoyd, Cauce, Takeuchi, & Wilson, 2000). Although this is the reason we chose to restrict this study to Caucasians, larger studies that can examine these associations among more diverse populations would be helpful. Additional limitations are that declines in health may contribute to declines in marital quality; there could also be reciprocal effects of marital quality and health over time. Longitudinal evidence indicates that low-quality marriages contribute to declines in self-rated health (Umberson, Williams, Powers, Liu, & Needham, 2006), but there have been no studies using measures of both biological indicators and marital quality indicators; therefore, we cannot assume the directionality of this association. Finally, like most studies, this study examined only one spouse in a spousal dyad, and therefore we were unable to examine the family unit. This is an important limitation in research on marriage because the marital quality rating of one spouse could influence the health of the other spouse.
Future research in this area should examine longitudinal changes in health indicators as a function of marital support in order to better understand the effects of different facets of marital quality over the life course. Marital quality should also be examined in more complex models of social relationships and as a potential moderator of the association between stress and inflammation (Uchino et al., 2008). Marital strain may exacerbate the effects of stress on inflammation or may have a direct effect on levels of inflammation; marital support may decrease the effects of stress on levels of inflammation. There is some indication that marriage is good for men regardless of the positive qualities of the marriage (i.e., a main effect), whereas marriage may be good for women only when they are satisfied in their marriage.
In sum, these findings suggest that marital support and strain are important factors to consider when examining the relationship between marriage and health, and also suggest that inflammation may be one mechanism through which marital quality influences health outcomes. Nevertheless, there are likely many biological pathways through which marital support and strain may influence health, and these other pathways are important to consider in future research.
Acknowledgments
We thank Jennifer Ailshire and Merril Silverstein for their helpful comments on earlier versions of this manuscript. The National Institute on Aging, Multidisciplinary Research Training in Gerontology (NIA, 5-T32-AG000037) provided generous support for this work. The original Midlife in the United States study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development, and the longitudinal follow-up study was supported by a grant from the National Institute on Aging (P01-AG020166). Support was also received from the following grants: 1UL1RR025011 (University of Wisconsin–Madison) from the Clinical and Translational Science Award, M01-RR00865 (University of California, Los Angeles), and M01-RR023942 (Georgetown) from the General Clinical Research Centers Program of the National Center for Research Resources, National Institutes of Health. The funding sources had no further role in the design, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. We thank the participants and their families for their trust, time, and dedication to this study, and the staff of the GCRCs for their support and dedication.
Contributor Information
Carrie J. Donoho, University of Southern California
Eileen M. Crimmins, University of Southern California.
Teresa E. Seeman, University of California, Los Angeles.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; 1991. [Google Scholar]
- Alley DE, Crimmins EM, Bandeen-Roche K, Guralnik J, Ferrucci L. Three-year change in inflammatory markers in elderly people and mortality: The Invecchiare in Chianti study. Journal of the American Geriatrics Society. 2007;55:1801–1807. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu P, Crimmins EM. Socioeconomic status and C-reactive protein levels in the U.S. population: NHANES IV. Brain, Behavior, and Immunity. 2006;20:498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Kessler RC. Everyday stressors and gender differences in daily distress. Journal of Personality and Social Psychology. 1998;75:670–680. doi: 10.1037//0022-3514.75.3.670. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Sommer KL. What do men want? Gender differences and two spheres of belongingness: Comment on Cross and Madson (1997). Psychological Bulletin. 1997;122:38–44. doi: 10.1037/0033-2909.122.1.38. discussion 51-5. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Social Science and Medicine. 2000;51:843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II study. American Journal of Epidemiology. 2007;165:828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carr D, Springer KW. Advances in families and health research in the 21st century. Journal of Marriage and Family. 2010;72:743–761. [Google Scholar]
- Choi H, Marks NF. Marital conflict, depressive symptoms, and functional impairment. Journal of Marriage and Family. 2008;70:377–390. doi: 10.1111/j.1741-3737.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. doi:10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Mahwah, N.J.: 2003. [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr. Social ties and susceptibility to the common cold. Journal of the American Medical Association. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: Findings from the health, aging and body composition study. Journal of the American Geriatrics Society. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proceedings of the National Academy of Sciences. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Vasunilashorn S, Kim JK, Alley D. Biomarkers related to aging in human populations. Advances in Clinical Chemistry. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JPT, Lennon L, Eiriksdottir G, Rumley A, Whincup P, Lowe GDO, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Medicine. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, Lee B, Khan M, Tacey R, Hill H, Celniker A. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharmaceutical Research. 2003;20:1885–1900. doi: 10.1023/b:pham.0000003390.51761.3d. [DOI] [PubMed] [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho CJ, Weigensberg MJ, Emken BA, Hsu JW, Spruijt-Metz D. Stress and abdominal fat: Preliminary evidence of moderation by the cortisol awakening response in Hispanic peripubertal girls. Obesity. 2010;19:946–952. doi: 10.1038/oby.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre ME, Meadows SO. Disaggregating the effects of marital trajectories on health. Journal of Family Issues. 2007;28:623–652. [Google Scholar]
- Dupre ME, Beck AN, Meadows SO. Marital trajectories and mortality among U.S. adults. American Journal of Epidemiology. 2009;170:546–555. doi: 10.1093/aje/kwp194. doi:10.1093/aje/kwp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging. Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. doi:10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A, D'Agostino R, Jr., Tracy RP, Haffner SM, Insulin Resistance Atherosclerosis Study Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- Finch CE. Inflammation in aging processes: An integrative and ecological approach. In: Masoro EJ, Austad SN, editors. Handbook of the biology of aging. 7th ed. Academic Press; London: 2011. pp. 275–296. [Google Scholar]
- Fincham FD, Linfield KJ. A new look at marital quality: Can spouses feel positive and negative about their marriage? Journal of Family Psychology. 1997;11:489–502. [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Ford ES, Loucks EB, Berkman LF. Social integration and concentrations of C-reactive protein among U.S. adults. Annals of Epidemiology. 2006;16:78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Sciences. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national probability sample (the MIDUS study). Psychosomatic Medicine. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Troxel WM, Kuller LH, Sutton-Tyrrell K, Edmundowicz D, Matthews KA. Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosomatic Medicine. 2003;65:952–962. doi: 10.1097/01.psy.0000097350.95305.fe. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Troxel WM, Matthews KA, Kuller LH. Marital status and quality in middle-aged women: Associations with levels and trajectories of cardiovascular risk factors. Health Psychology. 2003;22:453–463. doi: 10.1037/0278-6133.22.5.453. doi:10.1037/0278-6133.22.5.453. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GDO, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39:413–423. doi: 10.1017/S0033291708003723. doi:10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM, Levenson RW. Marital processes predictive of later dissolution: Behavior, physiology, and health. Journal of Personality and Social Psychology. 1992;63:221. doi: 10.1037//0022-3514.63.2.221. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Loving TJ, Malarkey WB, Stowell J, Houts C, Kiecolt-Glaser JK. Attachment avoidance predicts inflammatory responses to marital conflict. Brain, Behavior, and Immunity. 2009;23:898–904. doi: 10.1016/j.bbi.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Associations of very high C-reactive protein concentration with psychosocial and cardiovascular risk factors in an ageing population. Atherosclerosis. 2009;206:599–603. doi: 10.1016/j.atherosclerosis.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Waite LJ. Marital biography and health at mid-life. Journal of Health and Social Behavior. 2009;50:344–358. doi: 10.1177/002214650905000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: The national longitudinal mortality study. Annals of Epidemiology. 2000;10:224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Kronick RG. Marital status and longevity in the United States population. Journal of Epidemiology and Community Health. 2006;60:760–765. doi: 10.1136/jech.2005.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neuroscience and Biobehavioral Reviews. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: Newlyweds’ stress hormones foreshadow relationship changes. Journal of Consulting and Clinical Psychology. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. doi:10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: From basic science to medicine—40 years in immunology. Immunology. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnansky R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Annals of the New York Academy of Sciences. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB. Cardiovascular reactivity in cardiovascular disease: “Once more unto the breach.”. International Journal of Behavioral Medicine. 1994;1:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- März W, Winkler K, Nauck M, Böhm BO, Winkelmann BR. Effects of statins on C-reactive protein and interleukin-6 (the Ludwigshafen risk and cardiovascular health study). American Journal of Cardiology. 2003;92:305–308. doi: 10.1016/s0002-9149(03)00633-7. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Zheng H, Kravitz HM, Sowers MF, Bromberger JT, Buysse DJ, Owens JF, Sanders M, Hall M. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women? Study of women's health across the nation sleep study. Sleep. 2010;33:1649–1655. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McLoyd VC, Cauce AM, Takeuchi D, Wilson L. Marital processes and parental socialization in families of color: A decade review of research. Journal of Marriage and Family. 2000;62:1070–1093. [Google Scholar]
- Mezuk B, Diez Roux AV, Seeman T. Evaluating the buffering vs. direct effects hypotheses of emotional social support on inflammatory markers: The multi-ethnic study of atherosclerosis. Brain, Behavior, and Immunity. 2010;24:1294–1300. doi: 10.1016/j.bbi.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Dopp JM, Myers HF, Stevens SY, Fahey JL. Psychosocial predictors of natural killer cell mobilization during marital conflict. Health Psychology. 1999;18:262–271. doi: 10.1037//0278-6133.18.3.262. [DOI] [PubMed] [Google Scholar]
- Molloy GJ, Stamatakis E, Randall G, Hamer M. Marital status, gender and cardiovascular mortality: Behavioural, psychological distress and metabolic explanations. Social Science and Medicine. 2009;69:223–228. doi: 10.1016/j.socscimed.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES–D scale. Applied Psychological Measurement. 1977;1:385. [Google Scholar]
- Renshaw KD, Blais RK, Smith TW. Components of negative affectivity and marital satisfaction: The importance of actor and partner anger. Journal of Research in Personality. 2010;44:328–334. doi: 10.1016/j.jrp.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiology and Behavior. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Ross CE. Reconceptualizing marital status as a continuum of social attachment. Journal of Marriage and the Family. 1995;57:129–140. doi:10.2307/353822. [Google Scholar]
- Sbarra DA. Marriage protects men from clinically meaningful elevations in C-reactive protein: Results from the National Social Life, Health, and Aging Project (NSHAP). Psychosomatic Medicine. 2009;71:828–835. doi: 10.1097/PSY.0b013e3181b4c4f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster TL, Kessler RC, Aseltine RH., Jr. Supportive interactions, negative interactions, and depressed mood. American Journal of Community Psychology. 1990;18:423–438. doi: 10.1007/BF00938116. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Social ties and health: The benefits of social integration. Annals of Epidemiology. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis: Product of a primate adaptation. American Journal of Primatology. 2009;71:742–751. doi: 10.1002/ajp.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Research Reviews. 2010;9:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Gallo LC, Goble L, Ngu LQ, Stark KA. Agency, communion, and cardiovascular reactivity during marital interaction. Health Psychology. 1998;17:537–545. doi: 10.1037//0278-6133.17.6.537. [DOI] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, Berg CA, Florsheim P, Pearce G, Hawkins M, Henry NJM, Beveridge RM, Skinner MA, Ko KJ, Olsen-Cerny C. Conflict and collaboration in middle-aged and older couples: II. Cardiovascular reactivity during marital interaction. Psychology and Aging. 2009;24:274–286. doi: 10.1037/a0016067. doi:10.1037/a0016067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Gallo LC, Kuller LH. Marital quality and occurrence of the metabolic syndrome in women. Archives of Internal Medicine. 2005;165:1022–1027. doi: 10.1001/archinte.165.9.1022. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Frankel BG, Levin DM. Social support: Conceptualization, measurement, and implications for mental health. Research in Community and Mental Health. 1983;3:67–111. [Google Scholar]
- Uchino BN. What a lifespan approach might tell us about why distinct measures of social support have differential links to physical health. Journal of Social and Personal Relationships. 2009;26:53–62. doi: 10.1177/0265407509105521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Vaughn AA, Matwin S. Social psychological processes linking personality to physical health: A multilevel analysis with emphasis on hostility and optimism. In: Rhodewalt F, editor. Personality and social behavior. Psychology Press; New York: 2008. pp. 251–283. [Google Scholar]
- Umberson D, Williams K, Powers DA, Liu H, Needham B. You make me sick: Marital quality and health over the life course. Journal of Health and Social Behavior. 2006;47:1–16. doi: 10.1177/002214650604700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite LJ, Gallagher M. The case for marriage: Why married people are happier, healthier, and better off financially. Random House; New York: 2000. [Google Scholar]
- Waite LJ, Luo Y, Lewin AC. Marital happiness and marital stability: Consequences for psychological well-being. Social Science Research. 2009;38:201–212. [Google Scholar]
- Whisman MA, Sbarra DA. Marital adjustment and interleukin-6. Journal of Family Psychology. 2012;26:290–295. doi: 10.1037/a0026902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Umberson D. Marital status, marital transitions, and health: A gendered life course perspective. Journal of Health and Social Behavior. 2004;45:81–98. doi: 10.1177/002214650404500106. [DOI] [PMC free article] [PubMed] [Google Scholar]