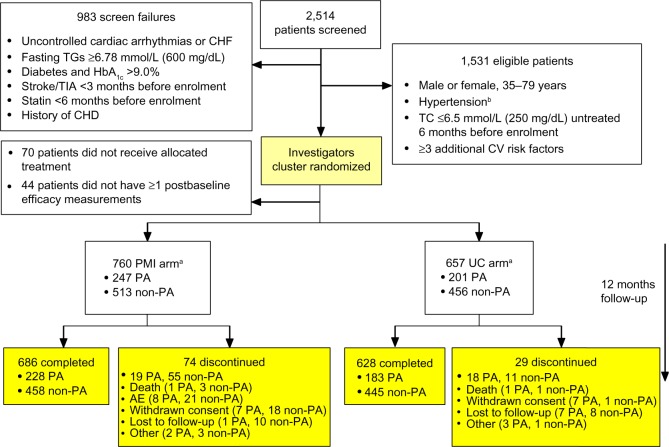
Figure 1.
Flow of PA and non-PA patients through the CRUCIAL trial.
Notes: aTreated patients with baseline and one or more postbaseline efficacy measurements. For one PMI investigator from a non-PA region, four out of 18 patients received UC during the treatment period and were analyzed in the UC arm for the safety population and in the PMI arm for the full analysis set; bhypertension: untreated: SBP ≥160 mmHg and/or DBP ≥100 mmHg; treated: SBP ≥140 mmHg and/or DBP ≥90 mmHg or diabetes: SBP >130 mmHg and/or DBP >80 mmHg.
Abbreviations: AE, adverse event; CHD, coronary heart disease; CHF, congestive heart failure; CRUCIAL, Cluster Randomized Usual Care versus Caduet Investigation Assessing Long-term-risk; CV, cardiovascular; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; PA, Pacific Asian; PMI, proactive multifactorial intervention; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; TIA, transient ischemic attack; UC, usual care.