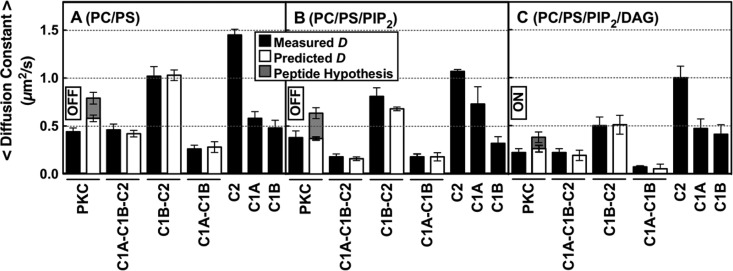
Figure 6.
Measured and calculated diffusion constants of full-length and truncated PKCα constructs on bilayers mimicking sequential lipid binding and kinase activation. On the plasma membrane, a PKCα molecule will typically encounter and bind PS, then PIP2, and then DAG based on the decreasing mole densities of these lipids (see the text). The indicated supported bilayer compositions (mole percents in Table 1) simulate this sequential binding by allowing protein equilibration first with PS (A), then with PS and PIP2 (B), and then with PS, PIP2, and DAG (C). Black bars are the average experimental diffusion constants of each construct on the indicated bilayer (Table 2 and Table S2 of the Supporting Information), determined by single-molecule TIRF analysis of at least 2500 diffusion tracks in at least five experiments (n ≥ 5). White bars are diffusion constants predicted by the new model (Figures 7 and 10A) for each multidomain construct on the indicated bilayer composition, calculated by combining the experimental diffusion constants of the domains proposed to contact the bilayer (eqs 1 and 2 and Table S2 of the Supporting Information). For contacts involving the C1A and/or C1B domain, calculations included a weighted average over the observed multiple states for these domains (Table S2 of the Supporting Information). Gray bars are diffusion constants predicted by replacing the C1A–bilayer friction of the new model (white bars) with pseudosubstrate peptide–bilayer friction, using the known friction of the tighter binding, more deeply penetrating MARCKS peptide60 as an upper limit on the friction expected for the N-terminal pseudosubstrate region. On each membrane type, this peptide hypothesis could not explain the observed diffusion constant, confirming that a C1 domain–membrane contact is necessary to explain the observed total friction. In all experiments, the free Ca2+ concentration was 6 μM in a physiological buffer at 22 ± 0.5 °C.