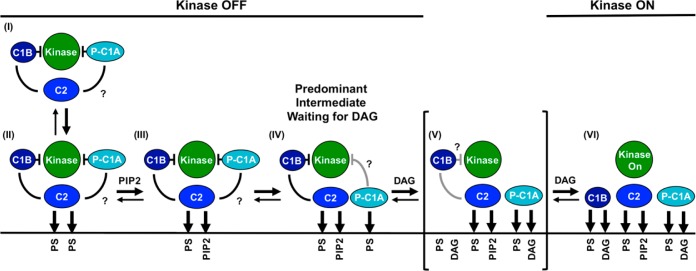
Figure 7.
Schematic model of the PKCα activation reaction showing interdomain and membrane contacts. Four of the indicated states [(II), (III), (V), and (VI)] are similar to those of a previous model,28 while a new, pre-DAG intermediate [(IV)] is proposed on the basis of new data. The new intermediate (IV) has been directly detected in single-molecule diffusion studies (see Results) and is the predominant intermediate awaiting the appearance of the activating DAG signal. The slow 2D diffusion of this intermediate arises from deployment of its C1A domain to the bilayer as indicated. Binding of DAG to this membrane-deployed C1A domain (IV) is proposed to trigger the formation of a transient state (V), not yet detected, in which binding of DAG to C1A may send a signal through the C1A–C1B linker to help dislodge the inhibitory C1B domain from the kinase domain and/or help guide C1B to the membrane. The resulting kinase on state is stabilized by the binding of a DAG molecule to C1B, thereby trapping its membrane-embedded state (VI). Lines between domains indicate inhibitory contacts (with a crossbar) or other protein–protein contacts; question marks indicate possible but not fully confirmed contacts. Gray contacts indicate a potential weakening of the interaction. A horizontal line represents a bilayer surface.