Abstract
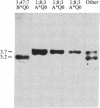
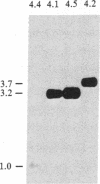
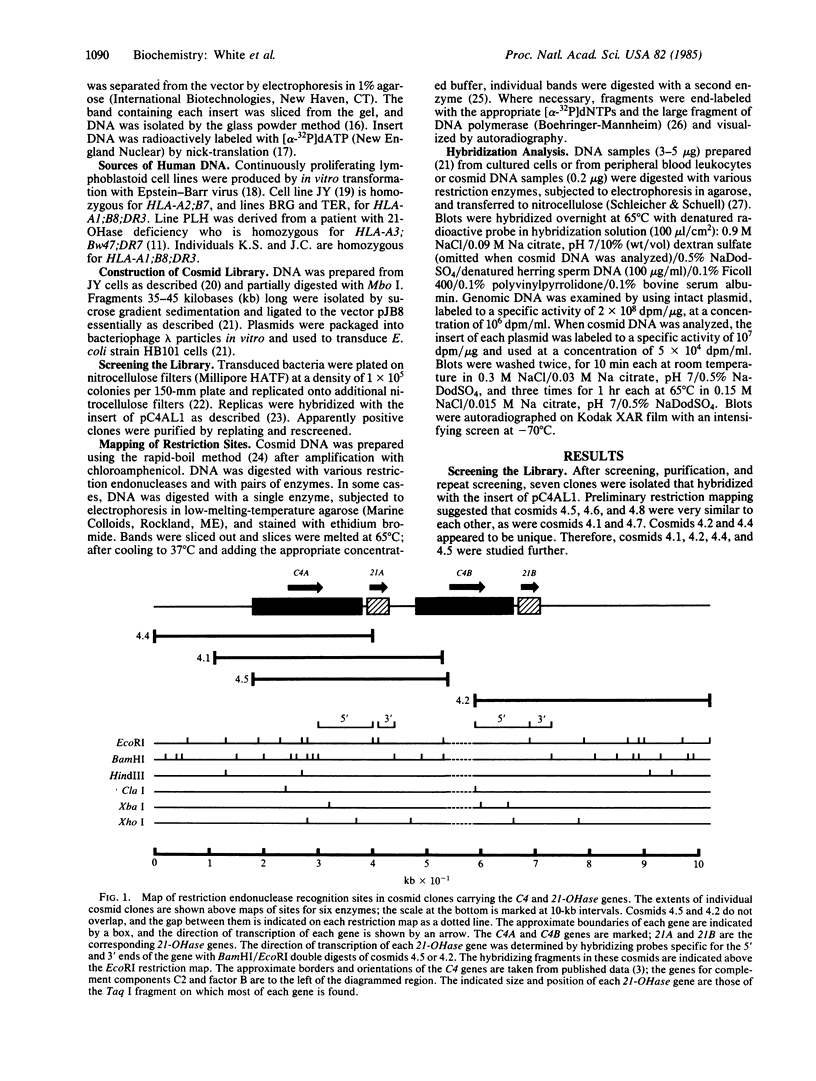
Two genes encoding steroid 21-hydroxylase [21-OHase; steroid 21-monooxygenase; steroid, hydrogen-donor: oxygen oxidoreductase (21-hydroxylating); EC 1.14.99.10], a cytochrome P-450 enzyme, have been located within the HLA major histocompatibility complex. Congenital adrenal hyperplasia due to 21-OHase deficiency is a common inherited disorder of cortisol biosynthesis which is in genetic linkage disequilibrium with certain extended HLA haplotypes. These haplotypes include characteristic serum complement allotypes. A series of cosmid clones was isolated from a human genomic library by using a probe encoding part of the fourth component of complement, C4. These clones also hybridized with a probe encoding most of human 21-OHase. Restriction mapping and hybridization analysis showed that there are two 21-OHase genes, each located near the 3' end of one of the two C4 genes. Hybridization with probes specific for the 5' and 3' ends of the 21-OHase gene showed that the 21-OHase and C4 genes all have the same orientation. The 21-OHase genes 3' to C4A and C4B carry T aq I fragments of 3.2 and 3.7 kilobases (kb), respectively. Both of these fragments are found in genomic DNA of most individuals. In DNA from an individual with the severe, "salt-wasting" form of 21-OHase deficiency who was homozygous for HLA-A3;Bw47;C4A*1;C4B*Q0(null); DR7, the 3.7-kb Taq I fragment is absent, whereas hormonally normal individuals homozygous for HLA-A1;B8;C4A*Q0;C4B*1;DR3 do not carry the 3.2-kb Taq I fragment. These data suggest that the 21-OHase "B" gene (3.7-kb Taq I fragment) is functional, but the 21-OHase "A" gene (3.2-kb Taq I fragment) is not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Raum D., Yunis E. J., Alper C. A. Extended HLA/complement allele haplotypes: evidence for T/t-like complex in man. Proc Natl Acad Sci U S A. 1983 Jan;80(1):259–263. doi: 10.1073/pnas.80.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Belt T., Palsdottir A., Porter R. R. Structure and organization of the C4 genes. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):379–388. doi: 10.1098/rstb.1984.0098. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J. Cloning of human mitochondrial DNA in Escherichia coli. J Mol Biol. 1980 Jun 15;140(1):15–34. doi: 10.1016/0022-2836(80)90354-x. [DOI] [PubMed] [Google Scholar]
- Dupont B., Oberfield S. E., Smithwick E. M., Lee T. D., Levine L. S. Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet. 1977 Dec 24;2(8052-8053):1309–1312. doi: 10.1016/s0140-6736(77)90362-2. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Nussenzweig V., Gigli I. Structural and functional differences between the H-2 controlled Ss and Slp proteins. J Exp Med. 1978 Nov 1;148(5):1186–1197. doi: 10.1084/jem.148.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. J., Dupont B., Pollack M. S., Levine L. S., New M. I. Complement C4 allotypes in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: further evidence for different allelic variants at the 21-hydroxylase locus. Clin Immunol Immunopathol. 1982 May;23(2):312–322. doi: 10.1016/0090-1229(82)90117-9. [DOI] [PubMed] [Google Scholar]
- O'Neill G. J., Yang S. Y., Dupont B. Two HLA-linked loci controlling the fourth component of human complement. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5165–5169. doi: 10.1073/pnas.75.10.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. J., Yang S. Y., Tegoli J., Berger R., Dupont B. Chido and Rodgers blood groups are distinct antigenic components of human complement C4. Nature. 1978 Jun 22;273(5664):668–670. doi: 10.1038/273668a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Seed B. Two-dimensional agarose gel electrophoresis "SeaPlaque" agarose dimension. Methods Enzymol. 1980;65(1):358–363. [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M. S., Levine L. S., O'Neill G. J., Pang S., Lorenzen F., Kohn B., Rondanini G. F., Chiumello G., New M. I., Dupont B. HLA linkage and B14, DR1, BfS haplotype association with the genes for late onset and cryptic 21-hydroxylase deficiency. Am J Hum Genet. 1981 Jul;33(4):540–550. [PMC free article] [PubMed] [Google Scholar]
- Raum D., Awdeh Z., Anderson J., Strong L., Granados J., Teran L., Giblett E., Yunis E. J., Alper C. A. Human C4 haplotypes with duplicated C4A or C4B. Am J Hum Genet. 1984 Jan;36(1):72–79. [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Silver L. M., Artzt K. Recombination suppression of mouse t-haplotypes due to chromatin mismatching. Nature. 1981 Mar 5;290(5801):68–70. doi: 10.1038/290068a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- White P. C., Chaplin D. D., Weis J. H., Dupont B., New M. I., Seidman J. G. Two steroid 21-hydroxylase genes are located in the murine S region. 1984 Nov 29-Dec 5Nature. 312(5993):465–467. doi: 10.1038/312465a0. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A. S., Goldberger G., Woods D. E., Markham A. F., Colten H. R. Use of a cDNA clone for the fourth component of human complement (C4) for analysis of a genetic deficiency of C4 in guinea pig. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5387–5391. doi: 10.1073/pnas.80.17.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman A. R., White R. A highly polymorphic locus in human DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6754–6758. doi: 10.1073/pnas.77.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]