Figure 1.
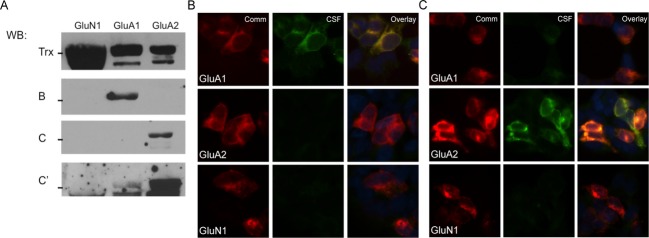
Patient CSF recognition of external domain fusion proteins is similar to staining of cells transfected with full subunits. (A) Patient CSF (blots B and C) recognizes a band of the correct size in transfected samples, indicating that native conformation may not be necessary for patient antibody recognition of AMPARs. GluA1, GluA2, and GluN1 all expressed well, with minimal proteolytic cleavage of the desired fusion protein when grown in pLyS BL21 Escherichia coli, as evidenced by blotting for the thioredoxin tag (Trx); even in this cell line, which minimizes protease activity, the GluA3 external domain fusion protein was almost entirely degraded during growth (data not shown). Two representative examples of patient reactivity are shown, as well as a darker exposure of the second patient to demonstrate minor GluA1 reactivity; all previously diagnosed anti-AMPAR encephalitis patients showed reactivity with fusion proteins. (B and C) CSF from two previously diagnosed anti-AMPAR encephalitis patients recognize GluA1 and/or GluA2-transfected HEK293 cells by immunocytochemistry in patterns that correspond to their observed GluA1/GluA2 external domain fusion protein reactivity (CSF from the same patient was used for (B) or (C)-labeled lanes in western blot in (A) and immunocytochemistry). (C) Example of one patient with primary GluA2 reactivity but minor GluA1 reactivity in transfected cells who showed the same differential reactivity with fusion proteins (A, bands C and C′; C, CSF panels). Comm, commercial antibody staining.
