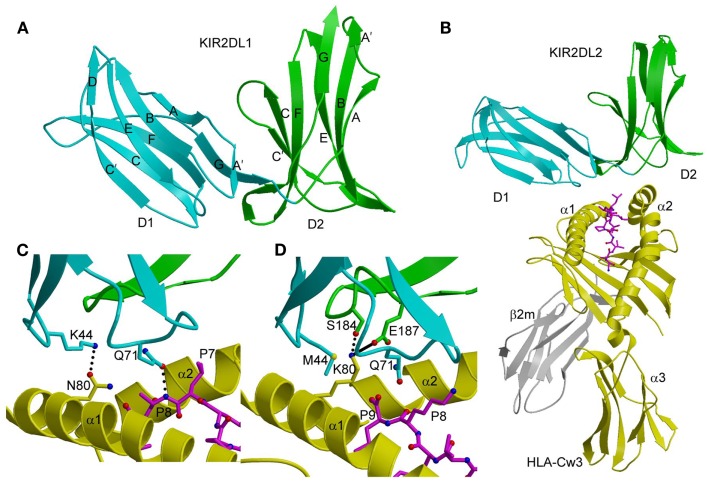
Figure 1.
Three-dimensional structures of KIR2DL and KIR2DL–HLA-C complexes. (A) Ribbon diagram of KIR2DL1 (PDB accession code 1NKR). The D1 domain is cyan; D2 is green. The secondary structural elements are labeled. (B) Ribbons diagram of KIR2DL2 bound to HLA-Cw3 (1EFX). The α1, α2, and α3 domains of the HLA-Cw3 heavy chain are yellow; β2m is gray; the peptide is magenta. (C) Basis for allelic specificity and peptide selectivity of KIR2D receptors. The dotted lines represent hydrogen bonds formed by Asn80 of HLA-Cw3 with Lys44 of KIR2DL2, and by Gln71 of HLA-Cw3 with P8 of the peptide. (D) Interactions of Lys80 of HLA-Cw4 (yellow) with specificity-determining residues of KIR2DL1 (D1 domain in cyan, D2 domain in green) in the KIR2DL1–HLA-Cw4 complex (1IM9). The solid line represents a salt bridge.