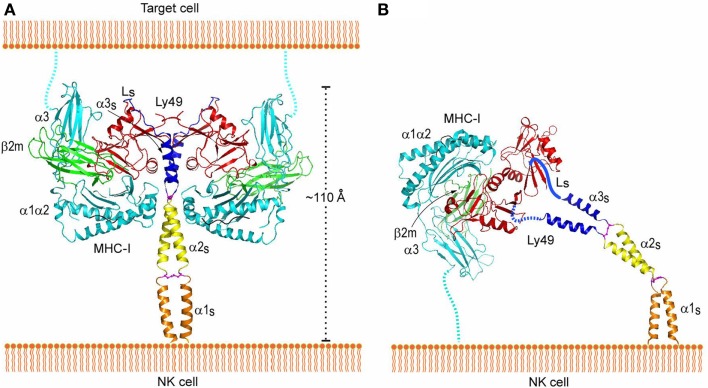
Figure 8.
Interaction of Ly49 receptors with MHC-I in trans and cis. (A) Trans interaction of an Ly49 receptor with two MHC-I molecules, based on the structures of Ly49L (3G8L) and the Ly49C–H-2Kb complex (3C8K). The α1, α2, and α3 domains of the MHC-I heavy chain are cyan; β2m is green; Ly49 NKD is red; helix α3S of the Ly49 stalk and loop LS connecting α3S to the NKD are blue; the disulfide bond linking the α3S helices is magenta. The predicted α1S and α2S helices of the stalk are orange and yellow, respectively, with the disulfide bond in magenta. The Ly49 homodimer on the NK cell binds two MHC-I molecules on the target cell. To bind in trans, the stalks must adopt a backfolded conformation, as the N-termini of the Ly49 monomers point away from the NK cell membrane (Ly49s are type II transmembrane proteins). (B) Cis interaction of Ly49 with MHC-I, based on the structure of Ly49L and the Ly49A–H-2Dd complex (1QO3). The LS loops connecting the α3S helices to the NKDs are drawn arbitrarily. The Ly49 homodimer binds one MHC-I molecule on the NK cell itself. In this case, the stalks must assume an extended conformation, as the N-termini of the Ly49 monomers point toward the NK cell. Reproduced with permission from Immunity (106).