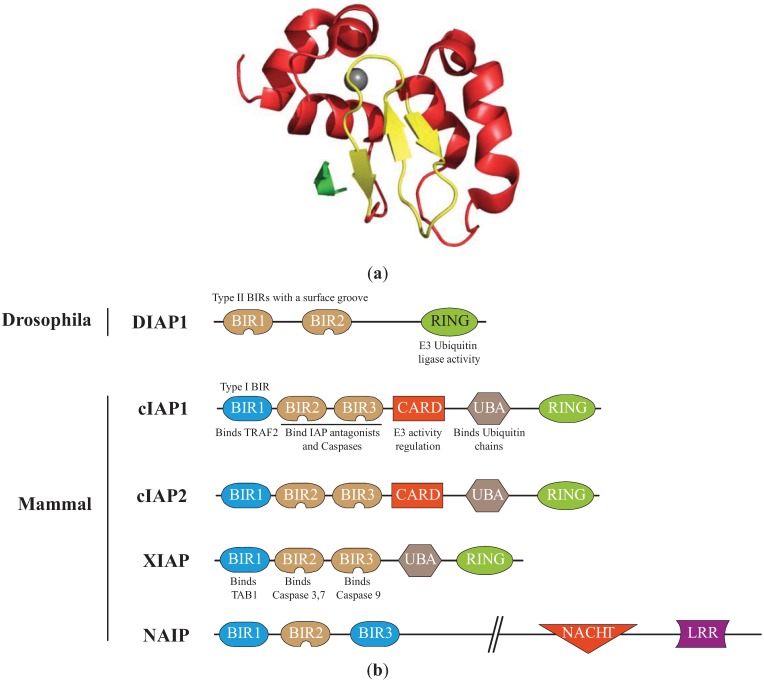
Figure 1.
Structure of the Inhibitors of apoptosis. (a) Structure of the cellular IAP1 (cIAP1)-baculoviral IAP repeat (BIR)3 bound to the caspase-9 N-terminal peptide [30]. BIR3 is organized in four α-helices (red) and 3 β-strand sheets (yellow) maintained by zinc ion (grey). The interaction involved the surface hydrophobic groove of cIAP1 and the N-terminal peptide (ATPFQ) of the caspase 9 sub-unit (Constructed using the PyMOL Molecular Graphics System). (b)Representation of IAPs involved in the regulation of apoptosis. The type I baculoviral IAP repeats (BIR, blue) of cIAPs and X-linked IAP (XIAP) can bind to cell signalling intermediates TNFR associated factor 2 (TRAF2) and Transforming Growth Factor beta-activated kinase 1-binding protein 1 (TAB1), respectively. The type II BIRs (brown) contain a surface hydrophobic groove allowing the interaction with IBM found in caspase sub-units and IAP antagonists. The ubiquitin Associated (UBA) domain binds ubiquitin chains. The caspase recruitment domain (CARD) is a module of regulation of the RING E3-ubiquitin ligase activity. The RING domain confers to IAPs an E3-ubiquitin ligase activity. NACHT (domain present in NAIP, CIITA, HETE and TP1). LRR: Leucine Rich Repeat.