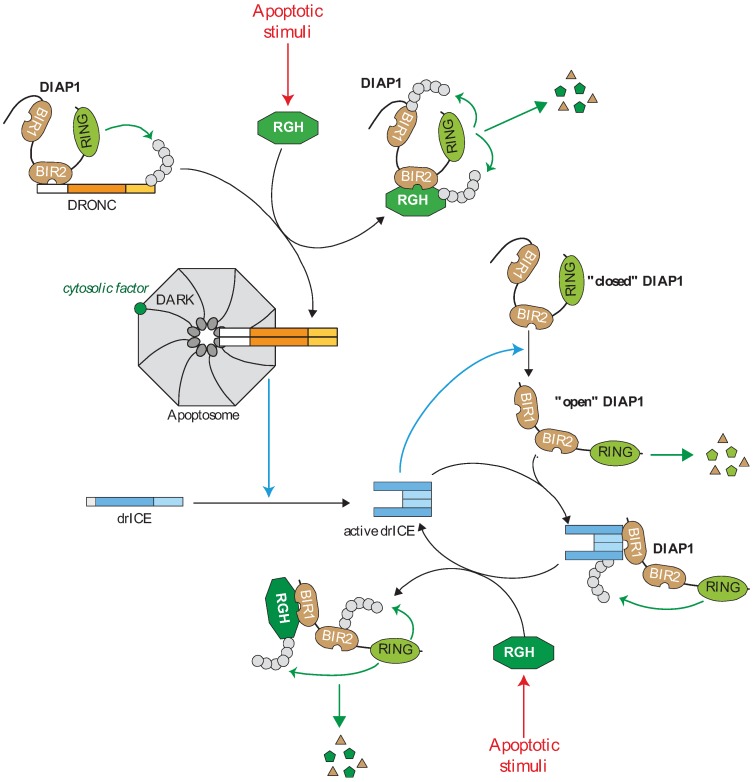
Figure 2.
Regulation of the caspase cascade by IAPs in drosophila. In living cells, the caspase activating cascade is maintained in check by a direct interaction of caspases with the Drosophila IAP1 (DIAP1). The DIAP1 BIR2 binds to the prodomain of the apoptotic initiator DROsophila Nedd-2-like Caspase (DRONC) and the RING induces DRONC ubiquitination preventing apoptosome assembly. DIAP1 is expressed in “closed conformation” in which the N-terminal sequence hides BIR1 surface groove. Effector caspase mediates the cleavage of the N-extremity of DIAP1 that releases the BIR1 domain which in turn interacts with the IBM exposed on the active form of effector drosophila melanogaster Interleukin-1-converting enzyme/Ced-3 related protease (drICE). DIAP1 inhibits drICE activity through a degradative or non-degradative ubiquitination or neddylation. The “open” form of DIAP1 is highly unstable and rapidly degraded by the N-end-rule-associated degradation machinery. Apoptotic stimuli induce the expression of the IBM-carrying IAP antagonists Reaper, Grim or Hid (RGH) wich strongly bind and neutralize DIAP1 through IBM-BIRs interaction. The IAP antagonist-DIAP1 interaction promotes DIAP1-autoubiquitination and degradation.