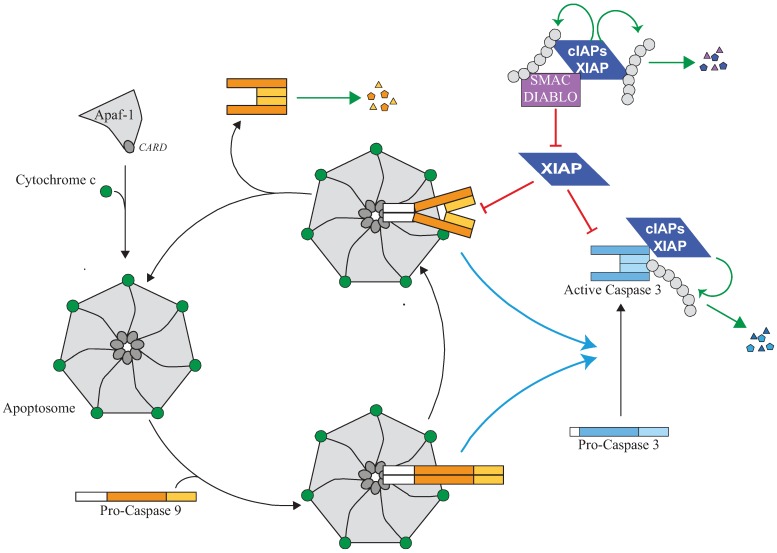
Figure 3.
Regulation of the apoptosome and caspase activity by IAPs. The release of cytochrome-c from mitochondria which occurs during intrinsic pathway of apoptosis triggers an ATP-dependent conformational change and oligomerisation of the adaptor apoptotic protease activating factor 1 (Apaf-1) in a heptameric complex apoptosome. Apaf-1 then recruits Caspase-9 via its pro-domain through a homotypic CARD-CARD interaction. Caspase-9 is activated by homodimerisation and promotes the activating cleavage of effector caspase-3 leading to apoptosis. Caspase-9 undergoes autocatalytic processing and is then quickly disconnected from the apoptosome which is free to recruit a new pro-caspase-9. XIAP can control caspase activating pathway at several steps. First, XIAP is present in the apoptosome where it directly binds processed caspase-9 and inhibits its activity. The inhibition of caspase-9 by XIAP could stabilize the caspase-9 apoptosome complex and block the cycle of caspase-9 activation. Second, XIAP can directly bind and inhibit active effector caspase-3. XIAP can inhibit caspase activity by hindering substrate accessibility or hiding the protease catalytic residue, and/or by promoting ubiquitination or neddylation. Although unable to inhibit their activity, cIAP1 can bind to processed caspases and promote their ubiquitination. An IBM-dependent binding of IAP antagonists such as Smac/Diablo prevents XIAP-mediated caspase inhibition while cIAPs could interfere with neutralizing binding XIAP-Smac/Diablo.