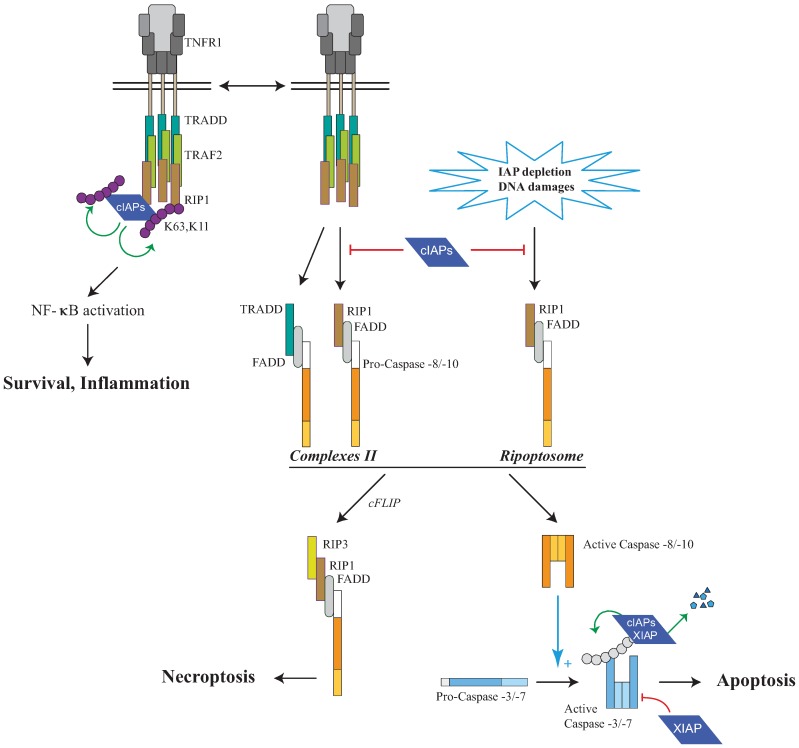
Figure 4.
Regulation of RIP1-containing platforms by IAPs. Tumor Necrosis Factor Receptor 1 (TNFR1) stimulation induces the recruitment, to the receptor, of cIAPs and Receptor interacting protein 1 (RIP1) via the adaptors TNFR1-associated Death domain (TRADD) and TNFR associated factor (TRAF2). cIAPs trigger K63 self-ubiquitination and K11 and K63-ubiquitination of RIP1 leading to NF-κB activation and survival. In the absence of cIAPs, a secondary RIP1-containing cytoplasmic complex is formed (complex II) leading to cell death. A cytoplasmic RIP-1-containing complex named RIPoptosome can also be assembled, in the absence of Death Receptor stimulation, after DNA-damage-mediated IAP depletion or the use of synthetic IAP antagonists known to induce IAP degradation. RIP1-containing platform can lead to either caspase-8 or -10 activation and apoptosis, or caspase-independent cell death referred to as Necroptosis. Initiator caspases-8 or -10 induce the activating proteolytic processing of effector caspase-3 or -7 responsible for apoptosis. IAPs can control cell death at different levels: (1) cIAPs can induce the K63 and K11 ubiquitination of RIP1 allowing NF-κB activation and preventing the formation of complex II or RIPoptosome; (2) cIAPs and XIAP can induce K48-ubiquitination of RIP1 leading to its proteasomal degradation. (3) XIAP can directly inhibit the activity of processed effector caspase-3 or -7; (4) cIAPs and XIAP can induce ubiquitination and proteasome-mediated degradation of processed forms of caspase-3 or -7.