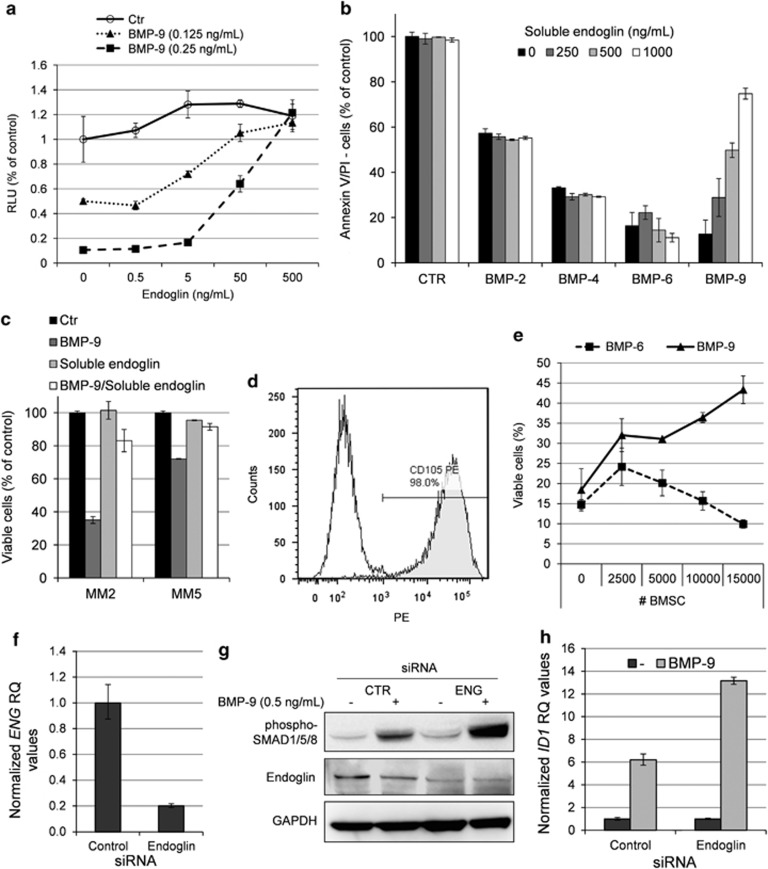
Figure 3.
Endoglin inhibited BMP-9, but not BMP-2, BMP-4 or BMP-6. (a) INA-6 cells were treated for 3 days with indicated concentrations of BMP-9 in the presence of increasing amounts of recombinant human soluble endoglin. Relative cell growth was measured using the CellTiter-Glo assay. (b) IH-1 cells were treated for 3 days with BMP-2 (200 ng/ml), BMP-4 (20 ng/ml), BMP-6 (300 ng/ml) or BMP-9 (5 ng/ml) in the presence of increasing amounts of soluble endoglin. Cell viability was measured by annexin V and PI staining. (c) Myeloma cells from patient MM2 and MM5 were treated with BMP-9 (5 ng/ml), endoglin (1 μg/ml) or a combination of both for 3 days before cell viability was measured using annexin V and PI. Untreated cells were used as control. (d) BMSCs were incubated with PE-labeled endoglin or isotype control antibodies and analyzed by flow cytometry. (e) INA-6 cells were grown in coculture with increasing numbers of BMSCs in the presence of BMP-9 (0.25 ng/ml) or BMP-6 (50 ng/ml). Cell membrane integrity was evaluated as a measure of viability using the YO-PRO-1 DNA stain as described in the Materials and Methods section. (f) QRT-PCR showing knockdown of endoglin (ENG) mRNA in siRNA-treated CAG cells. The cells were transfected with either Non-targeting Control or ENG siRNA and cells were collected 2 days post transfection. (g) Transfected CAG cells were treated 2 days post transfection with BMP-9 (0.5 ng/ml) for 1 h and subjected to immunoblotting with antibodies detecting phosphorylated SMAD1/5/8, endoglin or GAPDH. (h) CAG cells treated as in (f) and analyzed for expression of ID1 mRNA by QRT-PCR. Error bars represent 1 s.d. RQ, relative quantitation.