Figure 2. Overview of dynein composition.
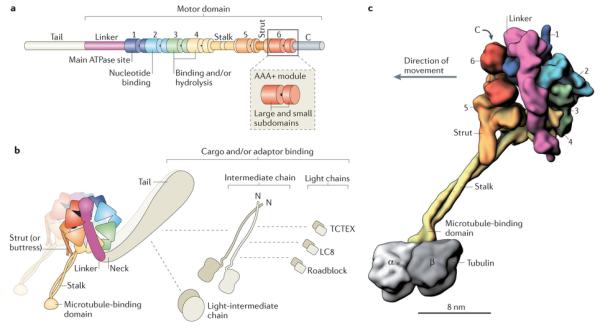
a | Linear representation of domains within the dynein heavy chain. The amino-terminal tail domain is involved in dynein oligomerization, cargo-binding and regulation, but is not part of the minimal motor domain capable of producing movement in vitro. The motor domain comprises the linker domain, six AAA+ modules (1–6), the coiled-coil stalk and strut, and the carboxy-terminal region. The motor domain of Dictyostelium discoideum dynein has a molecular mass of ~380 kDa. In many fungal dyneins, the C-terminal region is shorter and the motor domain is ~330 kDa. Each of the AAA+ modules is composed of a large N-terminal subdomain and a smaller C-terminal subdomain (see inset and Box 2). b | The cytoplasmic dynein complex contains a pair of identical heavy chains. Within each heavy chain, the six AAA+ modules fold into a ring. The stalk protrudes as an extension from the small subdomain of AAA4. The tail is connected to AAA1 by the linker domain, which arches over the AAA+ ring. In Chlamydomonas reinhardtii inner arm dynein-c, a sharp ~90° kink exists between the linker and the tail90, 92, 115. Although not yet visualized in cytoplasmic dynein, a similar kink might exist, as it would prevent a steric clash between the tail and the microtubule. In dynein-c, this neck region of the tail is a natural site of flexibility in the molecule, allowing the angle of the tail to vary with respect to the motor domain90, 92, 115. The cytoplasmic dynein heavy chains assemble with up to five types of associated subunit, which are also dimers148. The associated subunits comprise the intermediate chain, the light-intermediate chain and three classes of light chain: TCTEX, LC8 and Roadblock. Dashed lines indicate reported interactions of the associated subunits with each other and with the tail26. The three-dimensional (3D) arrangement of the associated subunits with respect to the tail is unknown. c | A 3D model of the cytoplasmic dynein motor domain bound to the microtubule (the associated subunits are not shown). As no high-resolution structure currently exists for the entire motor domain bound to a tubulin dimer, this model is based on a 2.8 Å crystal structure of the D. discoideum dynein motor domain lacking the microtubule-binding domain93 (Protein Data Bank ID: 3VKG), joined to a cryo-electron microscopy-derived model of the mouse microtubule-binding domain bound to an α-tubulin–β-tubulin dimer112 (Protein Data Bank ID: 3J1T). Subdomains are shown in surface representation, with the two long α-helices in the stalk rendered separately to emphasize their coiled-coil arrangement. The six AAA+ modules are numerically labelled.
