Figure 3. Model of the mechanochemical cycle of a cytoplasmic dynein motor domain.
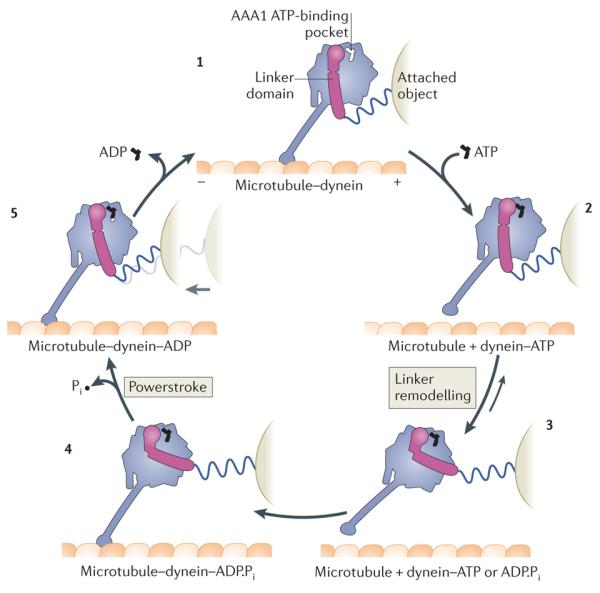
Model for the Dictyostelium discoideum cytoplasmic dynein motor domain85, 86, 91, 93, 111, 118. Plus and minus signs indicate microtubule polarity. The dynein motor domain moves towards the minus end of the microtubule. Force exerted on an attached object is shown schematically by the stretching of a spring, which does not represent a part of the dynein structure. Conceptually, the attached object could represent a partnering motor domain in a cytoplasmic dynein dimer or the cargo microtubule of an axonemal dynein. With no nucleotide bound at the AAA1 ATPase site, the dynein motor domain is tightly bound to the microtubule (1). ATP binding induces rapid dissociation from the microtubule (2) (Fig. 4). The dissociation rate is ~460 s–1 for the D . discoideum dynein motor domain85. A slower ATP-driven change (~200 s–1) is the remodelling of the linker domain85, which is displaced across the AAA+ ring (3) (Fig. 5). Remodelling of the linker extends the search range of the microtubule-binding domain along the microtubule. After hydrolysis of ATP to ADP and inorganic phosphate (Pi), the motor domain is thought to engage a new binding site on the microtubule, initially via a weak interaction (4). Strong binding to the microtubule accelerates the release of Pi from AAA1, inducing the linker to revert to its straight form. This transition is speculated to represent the powerstroke: the main step in which force (indicated by the light grey arrow) is transmitted to the attached object (5). Finally, ADP is released from AAA1 and the cycle restarts. The occupancy of other nucleotide-binding AAA+ modules of dynein, AAA2, AAA3 and AAA4, during the cycle, is unknown. Although the cycle is shown starting with dynein having no nucleotide bound at AAA1, it is not meant to imply that this is the longest-lived state. Indeed, in vivo, where the ATP concentration is in the millimolar range, ATP would rapidly bind at AAA1 once ADP is released, and thus this state would be populated only transiently.
