Figure 4. Cyclic microtubule binding.
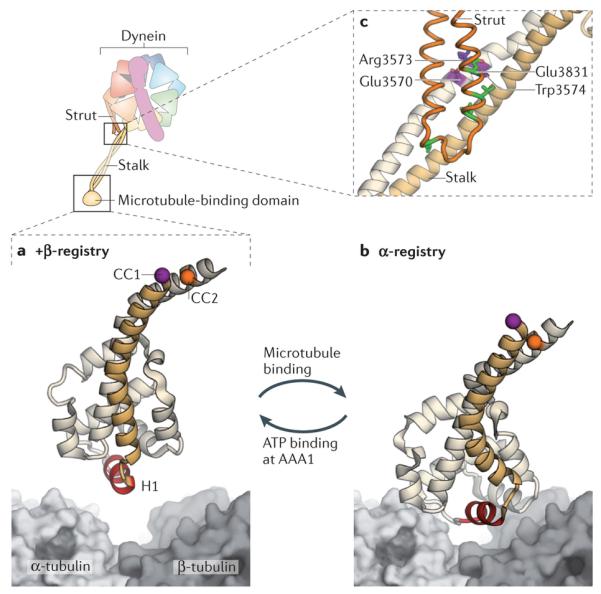
A cartoon of the dynein motor domain is shown, with regions of interest enlarged in parts a–c. a | Crystal structure of the microtubule-binding domain from mouse cytoplasmic dynein in its weak binding state110 (Protein Data Bank (PDB) code: 3ERR), shown above a surface representation of a tubulin dimer. b | Model of the mouse microtubule-binding domain strongly bound to the tubulin dimer, based on a ~10 Å cryo-electron microscopy (cryo-EM) map and steered molecular dynamics112 (PDB code: 3J1T). The binding site of dynein between α-tubulin and β-tubulin overlaps with that of kinesin149. Although the positioning of atoms in the cryo-EM derived model of the microtubule-binding domain is less certain than in the crystal structure, it is clear that helix H1 undergoes a large displacement on forming a strong interface with the microtubule112. Movement of H1 is thought to be associated with changes in the relative alignment or registry of the α-helices (CC1 and CC2) of the stalk, thereby allowing the microtubule-binding domain and the AAA+ ring to communicate through the stalk. This communication is vital for dynein movement, as it allows the microtubule-binding domain to sequentially bind and release its track during the ATPase cycle (see Fig. 3). A marker on CC1 (purple) and CC2 (orange) is coloured in each panel to highlight the registry change between the models. c | Communication between the AAA+ ring and the microtubule-binding domain depends critically93 on interactions between the stalk (an outgrowth of AAA4) and the strut (an outgrowth of AAA5). These stalk–strut interactions are expected to change during the ATPase cycle but, thus far, they have only been visualized in the ADP-bound state of the Dictyostelium discoideum dynein motor, in which the stalk adopts the α-registry93. Four highly conserved residues on the strut (Glu3831, Leu3835, Leu3838 and Leu3846; green) and a highly conserved triad of residues on CC2 of the stalk (Glu3570, Arg3573 and Trp3574; purple) might be important for the structure and dynamics of the stalk–strut interface.
