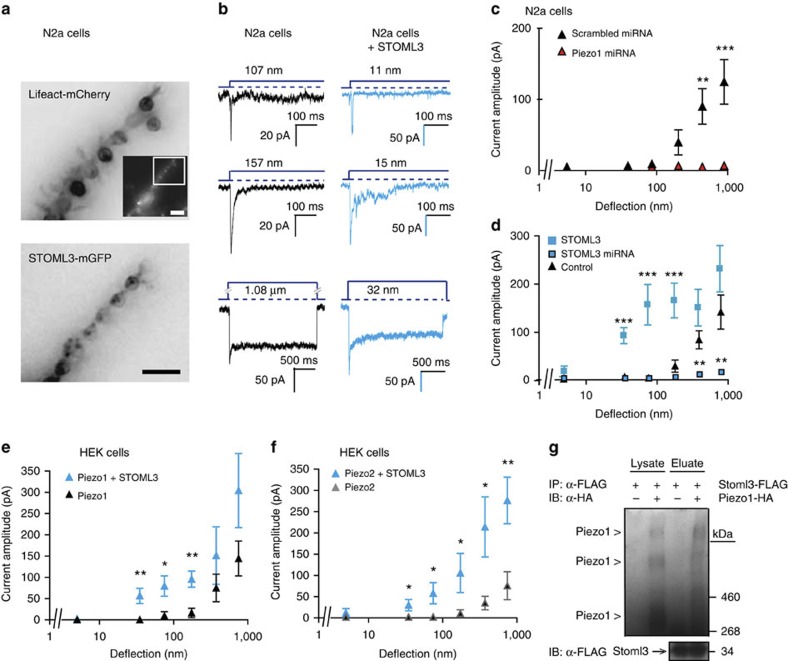
Figure 5. STOML3 increases Piezo1 and Piezo2 sensitivity.
(a) Inverted epifluorescence images of N2a neuroblastoma cells expressing Lifeact-mCherry and STOML3-mGFP cultured on uncoated, PDMS pillar arrays (similar observations were made in 26 cells from 7 transfections (lifeAct) and in 19 cells from 10 transfections (STOML3-mGFP)). Inset is an overview of Lifeact-mCherry signal in an individual cell; scale bars, 10 μm. (b) In individual cells, mechanically gated currents with variable kinetics were observed: black traces N2a control cells; blue traces N2a cells overexpressing STOML3-mGFP. (c–f) Stimulus–response data were binned and weighted by cell, and displayed as mean±s.e.m. and compared using Student’s t-test where *P<0.05, **P<0.01, ***P<0.001. (c) When endogenous Piezo1 was knocked down with miRNA (100 measurements, 10 cells) mechanosensitivity was significantly reduced compared with control cells treated with scrambled miRNA (145 measurements, 12 cells), Two-way analysis of variance (P<0.001), with Bonferroni post-tests (**P<0.01). (d) Current amplitudes were detected with stimuli much less than 100 nm in N2a cells overexpressing STOML3-mGFP (n=19 cells) compared with control cells (n=26 cells), and knockdown of endogenous STOML3 messenger RNA led to a strong reduction in current amplitudes below control levels (**P<0.01; data compared with miRNA controls plotted in panel c). (e,f) Stimulus–response data of mechanically gated currents in HEK-293 cells expressing Piezo1 (e) or Piezo2 (f) in the presence or absence of STOML3. As seen in N2a cells, the presence of STOML3 dramatically increased Piezo channel-mediated mechanosensitivity in HEK-293 cells. (g) Co-immunoprecipitation of Piezo1 with STOML3 pulldown in HEK-293 cells. Experiment was repeated six times, and in each case bands corresponding to Piezo proteins were detected in eluates from STOML3 pulldown.