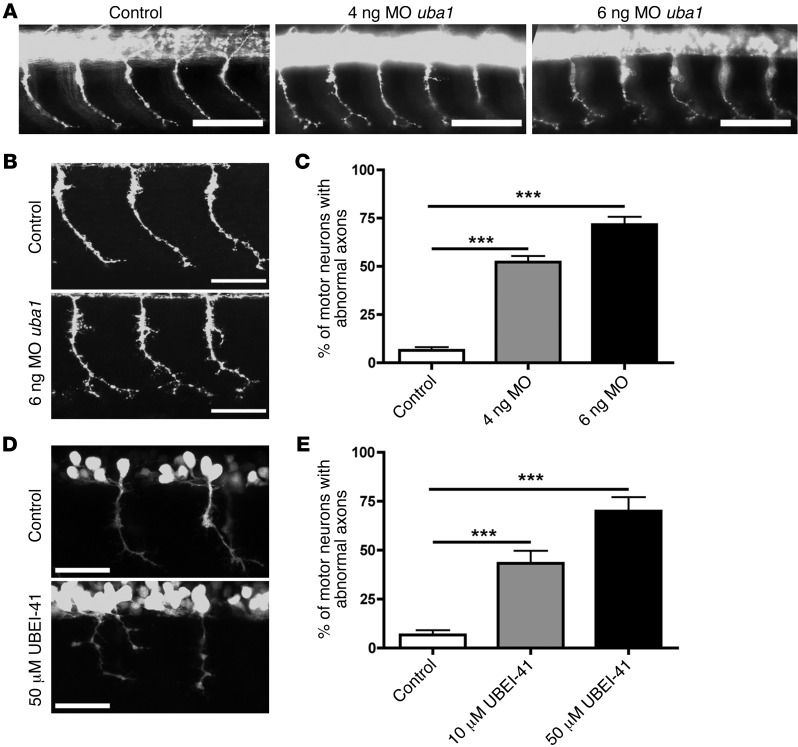
Figure 3. Genetic and pharmacological suppression of uba1 in zebrafish leads to dose-dependent motor axon pathology.
(A) Representative fluorescence micrographs of motor axons growing out from the spinal cord in a control zebrafish 34 hours after fertilization, and in animals injected with either 4 ng or 6 ng of a MO suppressing uba1 levels (see Supplemental Figure 4). (B) Representative higher-magnification confocal micrographs showing abnormal sprouts and axonal extensions in motor axons from MO-treated zebrafish. Scale bars: 50 μm. (C) Dose-dependent increase in the occurrence of abnormal branching in MO-treated zebrafish (Kruskal-Wallis test with Dunn’s post hoc test; uninjected, n = 310 motor neurons, n = 31 animals; 4 ng, n = 360, n = 36 animals; 6 ng, n = 360, n = 36 animals). Only motor axons with modest (type 2; see Supplemental Figure 4) or severely abnormal branching (type 3; see Supplemental Figure 4) were quantified as having abnormal branching. (D) Representative confocal micrographs showing perturbations in motor axon morphology in Tg(hb9:gfp) zebrafish embryos treated with 50 μM of the UBA1 inhibitor UBEI-41. Note the presence of a “double-exit” motor axon (right hand side of image) in the UBEI-41 example, with the axon branch emerging on the right side of the pair showing stunted outgrowth. Scale bars: 100 μm; 30 μm (B, D). (E) Levels of abnormal motor axon branching and axon outgrowth in UBEI-41–treated zebrafish. Note the dose-dependent increase of numbers of aberrant motor axons in the UBEI-41 group compared with DMSO controls (10 μM UBEI-41 n = 258 nerves, n = 11 animals; 50 μM UBEI-41 n = 280 nerves n = 12 animals; Kruskal-Wallis test with Dunn’s post hoc test). ***P < 0.001.