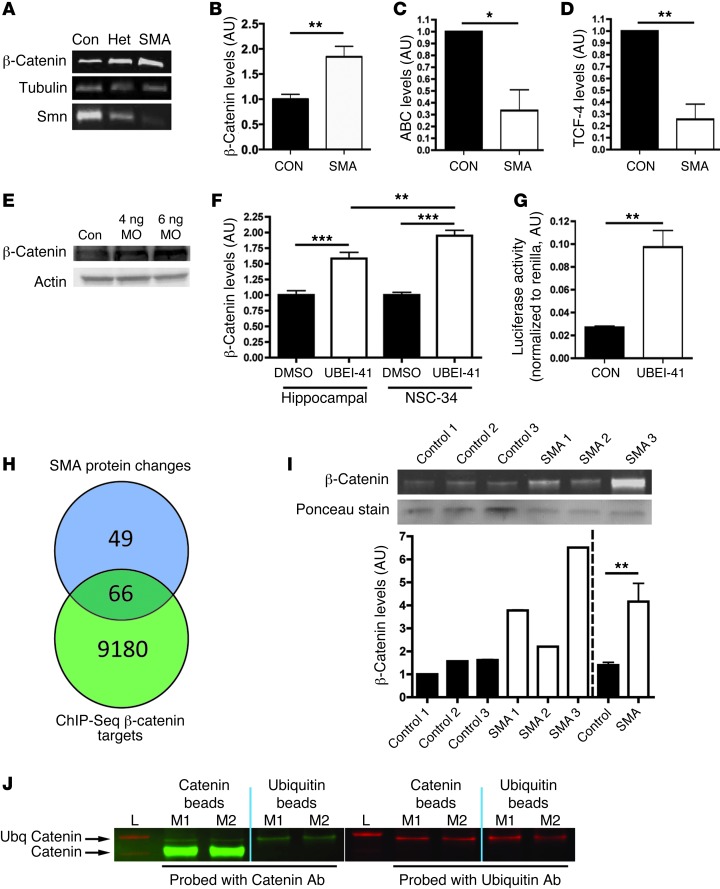
Figure 4. β-Catenin is a downstream target of UBA1 and accumulates in SMA.
(A) β-Catenin and SMN protein in spinal cord of severe SMA, heterozygous (Het), and littermate (con) mice at P5 (tubulin: loading control). (B–D) β-Catenin was increased in P10 Taiwanese SMA mouse spinal cord (n = 3 control mice, n = 4 SMA; unpaired 2-tailed t test), whereas stabilized β-catenin (ABC; C) and TCF-4, a β-catenin interacting protein required for activation (D), were both reduced (n = 3 CON, n = 3 SMA). (E) Increased β-catenin protein in zebrafish injected with 4 ng or 6 ng of a uba1 MO 48 hours after fertilization. (F) Increased β-catenin in rat hippocampal neurons and a motor neuron cell line (NSC-34) treated with 50 μM UBEI-41 (ANOVA with Tukey’s post-hoc test; n = 12 coverslips DMSO, n = 15 UBEI-41 hippocampal; n = 19 DMSO, n = 18 UBEI-41 NSC-34). (G) Increased β-catenin signaling activity in NSC-34 cells treated with 50 μM UBEI-41 measured with a luciferase reporter construct (n = 3 coverslips per treatment). (H) The majority of proteins modified in SMA synapses (66 out of 115 analyzed; see Supplemental Tables 1 and 2) are known β-catenin target genes identified by ChIP-Seq analyses. (I) Increased β-catenin protein in muscle biopsies from 3 human SMA patients (pooled data on right of dotted line). (J) Western blots for β-catenin (left panel; green) and ubiquitin (right panel; red) from co-IP experiments on synaptic extracts from 2 WT mice (L, ladder; M1, mouse 1; M2, mouse 2). Immunoblotting on the bound extract revealed the presence of ubiquitinated β-catenin (upper arrow). *P < 0.05; **P < 0.01; ***P < 0.001.