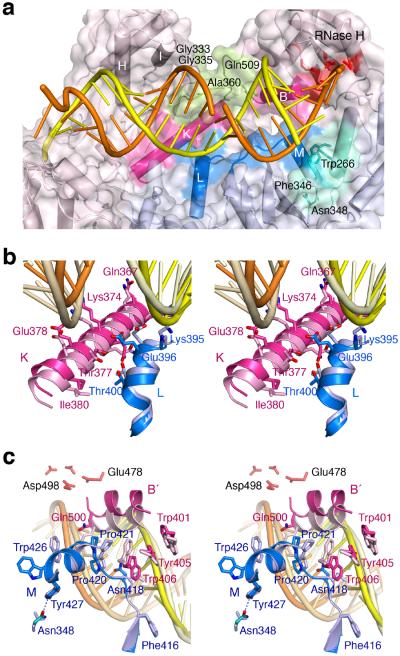
Figure 6.
The altered p51–p66 subunit interface interacts with the RNA/DNA hybrid. (a) Overview of the WT22Efv structure with the altered subunit interface highlighted in blue (p51) and pink (p66). NNRTI and NRTI resistance mutations36 are found in the p66 connection and RNase H domain (pea green) that contacts the widened major groove. Residues surrounding Asn348 in p51 and forming van der Waals contacts with the RNA strand are colored teal. (b) A close-up stereo view of the interfacial helices between the p51 (blue) and p66 (pink) connection domains. The altered protein interface relative to 1RTD (an RT–DNA ternary complex, in lighter shades) is correlated with the change from DNA binding (pale yellow and wheat) to RNA/DNA hybrid (orange/yellow) binding. (c) A close-up stereo view of the back of (a). This shows the p51 C-terminus (blue) and helix αB′ (pink) in the WT22Efv structure and in 3KK227 (an RT–DNA ternary complex complete with helix αM in p51, in lighter shades). The hybrid in the WT22Efv (orange and yellow) and DNA in 3KK2 are also shown. In panels (b) and (c), the p51 subunits are superimposed.