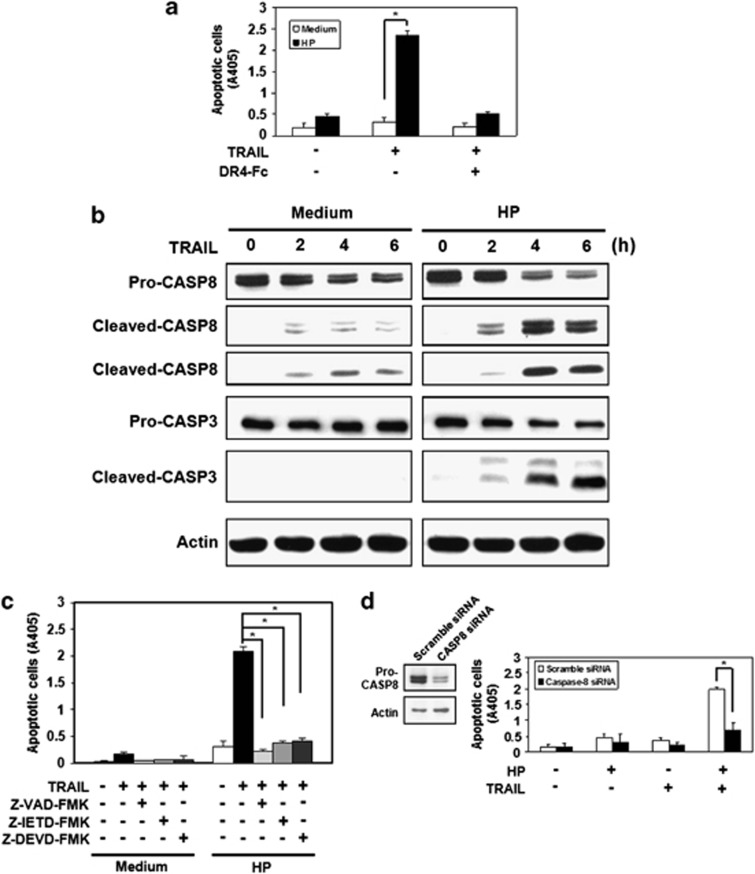
Figure 1.
H. pylori enhances sensitivity to TRAIL-mediated apoptosis in human gastric epithelial cell lines via activation of caspase-8 and its downstream pathway. (a) Human gastric epithelial cell line AGS were co-cultured with H. pylori for 12 h and incubated with recombinant TRAIL proteins in the presence or absence of soluble TRAIL receptor, DR4-Fc protein, for 12 h. Cell apoptosis was measured by cell death enzyme-linked immunosorbent assay (ELISA). Values represent means±S.D. of three independent experiments (*P<0.05). (b) AGS cells were co-cultured with H. pylori for 12 h and incubated with recombinant TRAIL proteins for the indicated times. Cell lysates were subjected to western blotting analysis for detection of caspase activation. The native forms of caspase-8 and caspase-3 are 55/54 and 32 kDa, respectively. The cleaved forms of caspase-8 are 43/41 and 18 kDa, respectively. The cleaved forms of caspase-3 are 19 and 17 kDa, respectively. The expression of actin serves as a loading control. CASP, caspase. (c) AGS cells were co-cultured with H. pylori for 12 h in the presence of pan-caspase inhibitor, Z-VAD-fmk (Bachem, Bubendorf, Sweden), specific caspase-8 inhibitor, Z-IETD-fmk, or specific caspase-3 inhibitor, Z-DEVD-fmk (Calbiochem, San Diego, CA, USA) and then were treated with recombinant TRAIL proteins for 4 h. Cell apoptosis was measured by cell death ELISA. Values represent means±S.D. of three independent experiments (*P<0.05). (d) AGS cells were transfected with scramble or caspase-8 siRNA, followed by incubating with H. pylori for 12 h and subsequently treated with TRAIL for 4 h. Cell apoptosis was measured by cell death ELISA. Values represent means±S.D. of three independent experiments (*P<0.05)