Figure 3.
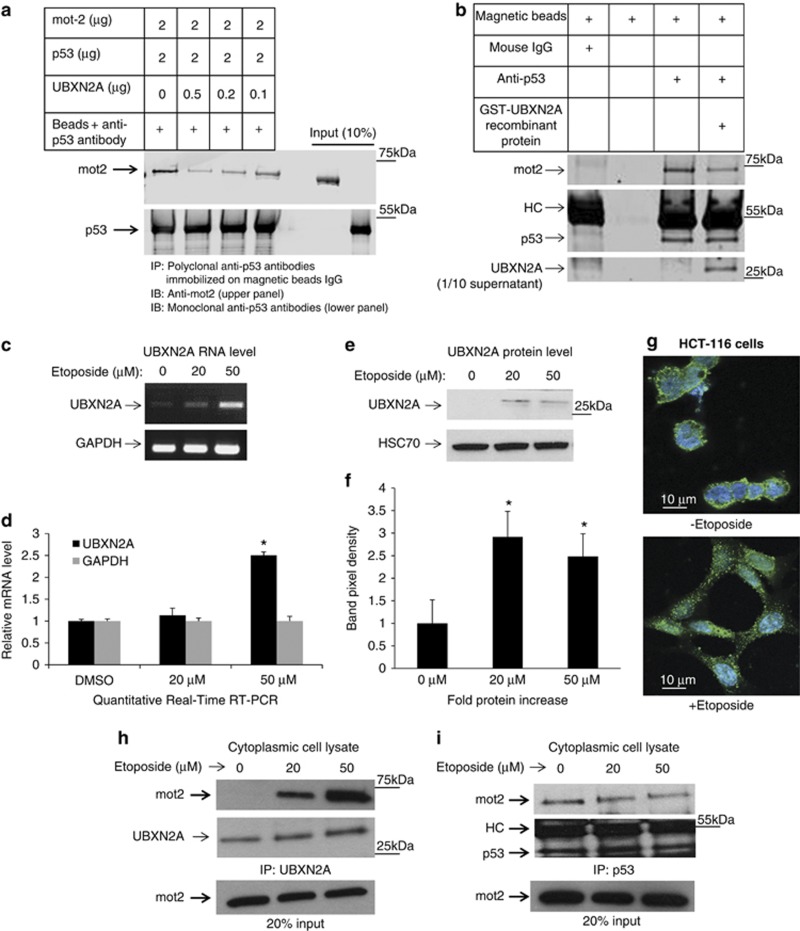
UBXN2A and p53 compete for binding to mot-2. (a) We investigated whether UBXN2A can decrease the association of p53 with mot-2 using an in vitro binding competition assay. First, recombinant human GST-p53 proteins bound to anti-p53 antibodies-IgG magnetic beads were incubated with human GST-mot-2 protein and increasing concentrations of human GST-UBXN2A recombinant proteins. Mot-2 proteins were eluted from the beads and analyzed by western blotting using an anti-mot-2 antibody. The same membrane was re-probed for p53 (lower panel) to show equivalent p53 in each IP. (b) The in vitro competition assay was further confirmed when the human GST-UBXN2A fusion proteins were incubated with cytosolic fractions enriched with mot-2 and p53 proteins (fractions 3-5, Figure 2e) of HCT-116 cells. The level of recombinant protein provided an ∼2.5:1 ratio of UBXN2A to endogenous mot-2. Cell lysates with and without UBXN2A were incubated with anti-p53 antibodies immobilized on magnetic Dynabeads protein G (Lanes 3 and 4). Beads with mouse IgG or beads alone were control groups in this experiment (lane 1 and 2). Western blotting showed that UBXN2A caused displacement of p53 binding from mot-2. These data support that UBXN2A and p53 compete for the mot-2. (c, d) A semiquantitative RT-PCR protocol (SuperScript III One-Step RT-PCR system) and quantitative real-time RT-PCR (d) showed etoposide enhances transcription of UBXN2A in HCT-116 cells. (e, f) At 24 h post-etoposide treatment (20 and 50 μM), total cell lysates were obtained, 50μg of protein was loaded in each lane, and the resulting blots were probed with an anti-UBXN2A antibody. UBXN2A's signal was normalized with the HSC70 signal (loading control). Together, these data confirmed UBXN2A expression is upregulated at the mRNA and protein levels upon genotoxic stress (P<0.05, n=3). DMSO was used as vehicle control. (g) Immunofluorescent staining reveals that UBXN2A shows a juxtanuclear staining characteristic of the ER/Golgi apparatus in the absence of etoposide. However, UBXN2A showed striking punctate cytoplasmic staining when HCT-116 cells incubated with etoposide (50 μM) for 24 h, suggesting a dynamic change in UBXN2A functional linkage networks in response to genotoxic stress. (h, i) The results presented in f indicate significant upregulation of UBXN2A in the cytoplasm with 20 and 50 μM etoposide after 24 h incubation. To verify whether this upregulated endogenous UBXN2A causes displacement of p53, we conducted a series of IP experiments. (h) Cytoplasmic lysates from e treated with no etoposide or 20 and 50 μM were subjected to IP using anti-UBXN2A (h) or anti-p53 (i) antibodies immobilized on magnetic IgG beads. Samples were resolved by 4–20% gradient SDS-PAGE and detected by WB using the indicated antibodies. The HC indicate the heavy immunoglobulin band. WB results indicate that UBXN2A binds to mot-2 in a etoposide dose-dependent manner, while the amount of mot-2 associated with p53 decreases simultaneously