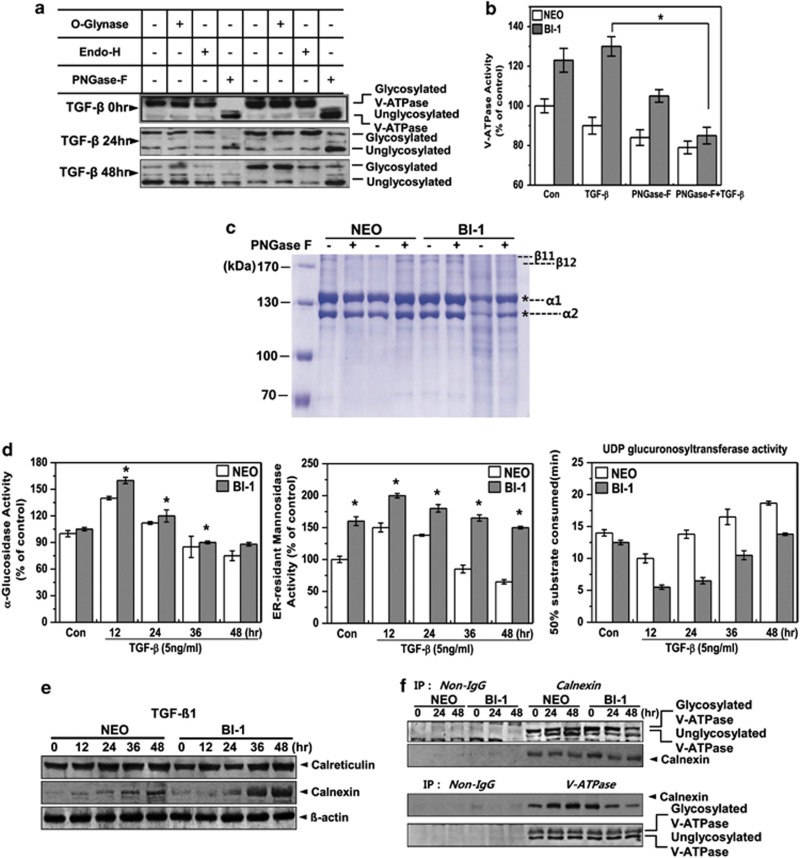
Figure 6.
BI-1 enhances N-glycosylation activity in the ER during TGF-β1 treatment. (a) Neo and BI-1 cells were cultured with 5 ng/ml TGF-β1 for 0, 24, or 48 h. O-glynase, Endo-H, or PNGase-F was added to the extracts followed by 30-min incubation. Immunoblotting was then performed with anti-V-ATPase antibody. (b) V-ATPase activity in the lysosome fractions isolated from 5 ng/ml TGF-β1-treated Neo or BI-1 cells in the presence or absence of PNGase-F was measured and quantified. *P<0.05, significantly different from TGF-β1-treated BI-1 cells. (c) Purified collagens from rat tails were incubated with lysosome fractions from TGF-β1-treated or non-treated Neo or BI-1 cells in the presence or absence of PNGase-F. Coomassie staining was performed as described in the Materials and Methods. * α1 or α2 collagen. (d) Neo and BI-1 cells were cultured in serum-free medium with 5 ng/ml TGF-β1 for 0, 12, 24, 36, or 48 h. After isolating a pure ER fraction, α-glucosidase, ER-resident mannosidase or UDP glucuronsyltransferase activity was analyzed as described in the Materials and Methods. *P<0.05, significantly different from Neo cells at each time point. (e) Neo and BI-1 cells were cultured in serum-free medium with 5 ng/ml TGF-β1 for 0, 12, 24, 36, or 48 h, and immunoblotting was performed with anti-calreticulin or calnexin antibodies. β-Actin antibody staining of the same loading was used as a control. (f) Neo and BI-1 cells were cultured in serum-free medium with 5 ng/ml TGF-β1 for 0, 24, or 48 h. Lysates were subjected to immunoprecipitation and immunoblotting with anti-V-ATPase or calnexin antibody