Fig. 1.
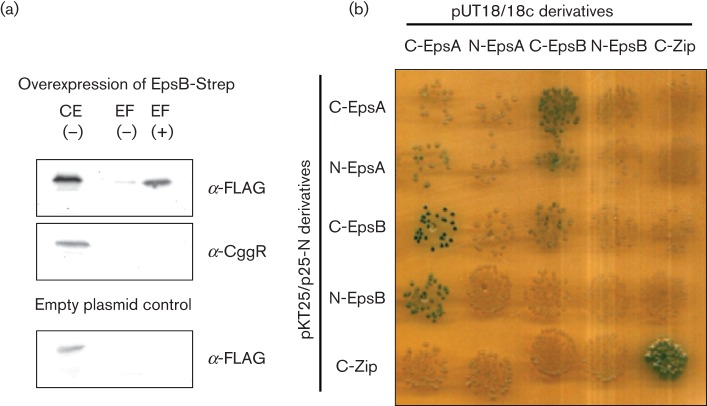
The tyrosine kinase EpsB and its cognate modulator protein EpsA interact physically. (a) EpsA co-purifies with EpsB. The EpsB-Strep fusion protein was expressed from plasmid pGP2126 under the control of a strong constitutive promoter in Bacillus subtilis GP1589 that expresses EpsA-FLAG under the control of its native promoter. To ensure high expression of EpsA-FLAG, the gene for the anti-activator SinR was deleted in this strain. Cells were grown in CSE-glucose medium until the late exponential growth phase. EpsB-Strep was purified in the absence (−) or presence (+) of the cross-linker formaldehyde. To detect the co-purified EpsA-FLAG protein, the elution fractions were heated to reverse the cross-linking and applied to a 12 % SDS polyacrylamide gel. After electrophoresis and blotting onto a PVDF membrane, EpsA was detected via its FLAG-tag. To test that the binding of EpsA to EpsB is not unspecific we tried to detect the CggR protein in the same elution fractions with a specific antibody. Additionally, we used an empty vector control to test unspecific binding of EpsA to the Streptactin column. CE, crude extract; EF, elution fraction. (b) EpsA and EpsB interact in the B2H system. The genes encoding EpsA and EpsB were cloned in the low-copy plasmids p25N and pKT25 and the high-copy plasmids pUT18 and pUT18c. These plasmids allow the expression of the genes of interest fused to the N or C terminus of the T18 or T25 domains of the Bordetella pertussis adenylate cyclase, respectively. The E. coli transformants harbouring both vectors were incubated for 48 h at 30 °C. Degradation of X-Gal and the resulting blue colour of the cells indicate interaction due to the presence of a functional adenylate cyclase.