Abstract
We respond to Valle and Clark,1 who assert that “conservation efforts may increase malaria burden in the Brazilian Amazon,” because the relationship between forest cover and malaria incidence was stronger than the effect of the deforestation rate.1 We contend that their conclusion is flawed because of limitations in their methodology that we discuss in detail. Most important are the exclusion of one-half the original data without a discussion of selection bias, the lack of model adjustment for either population growth or migration, and the crude classifications of land cover and protected areas that lead to aggregation bias.1 Of greater significance, we stress the need for caution in the interpretation of data that could have profound effects on regional land use decisions.
In a recent article, Valle and Clark1 found that, in 401 cities in the Brazilian Amazon, both deforestation and percent forest cover in a 20-km area around cities were associated with an increase in malaria cases reported to the primary health clinic in the city. Valle and Clark1 conclude that “conservation efforts may increase malaria burden in the Brazilian Amazon,” because the relationship between forest cover and malaria was stronger than the effect of the deforestation rate.1 We contend that their conclusion is flawed because of limitations in their methodology. Of greater significance, we stress the need for caution in the interpretation of data that could have profound effects on regional land use decisions. In stark contrast, Laporta and others2 conclude that biodiversity and intact forests can help eliminate local malaria transmission, consistent with our studies on malaria cases3 and risk.4,5 These opposing results show the need for additional discussion on this important issue, and we invite Valle and Clark1 to respond to our critique.
Valle and Clark1 use monthly malaria data collected over a 4.5-year period but use census data collected at a single time for their denominator to calculate malaria incidence. Furthermore, Valle and Clark1 confuse the term malaria incidence, the rate value typically used for epidemiological risk, with malaria cases, a statistic that fails to account for fluctuations in size of the population at risk; unfortunately, the latter was used in their risk estimates in figures 1 and 2 in ref. 1.
Urbanization and migration are dynamic processes that work across geographic and temporal scales to affect forest loss and disease rates.6,7 It has been recognized, specifically in Brazil, that as urban areas expand and improve infrastructure, malaria rates fall, consistent with the frontier malaria hypothesis—that malaria incidence particularly varies during early stages of urban development.8,9 In addition, migration, which is ubiquitous throughout the Brazilian Amazon,10 is a key determinant of malaria, because it alters the interface between humans and their environment. Migration has been used to understand the timing of frontier settlement, genetic variability of Plasmodium, and population age structure.7,10–12 Thus, to assume that population size and distribution remain static during a time period (2000–2010) when the Brazilian Amazon population increased by 23% (compared with 12% across Brazil) is extremely problematic.13 If population size was underestimated in areas with high forest cover, the results by Valle and Clark1 would be biased to a positive association between forest cover and malaria when, in fact, malaria case rates may have stayed constant or even declined. Although population growth may ultimately result in greater forest losses, to ignore a changing denominator in the calculation of a rate raises serious doubt about the validity of their conclusions.
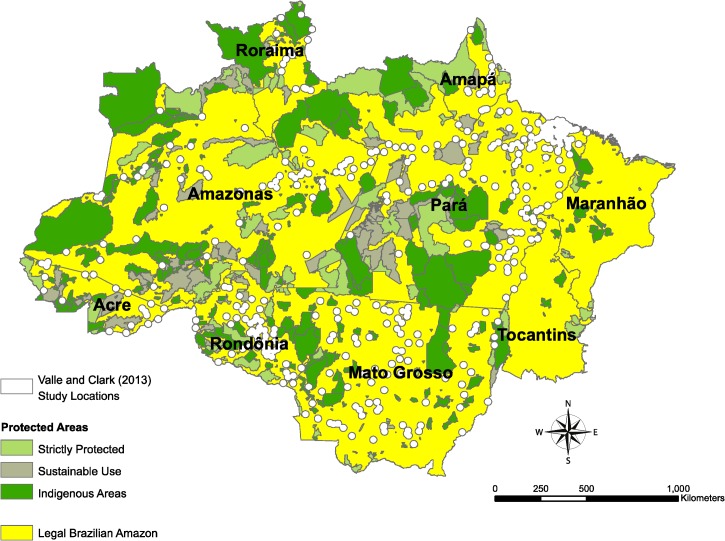
The process of data exclusion also poses challenges to inference. Valle and Clark1 excluded rural health facilities outside of established cities, cities with less than 2 years of malaria data, and the two easternmost states in the Amazon (Maranhao and Tocantins). Consequently, the resulting dataset contained only about one-half of the original malaria cases, and the 20-km buffer around their selected cities represents only 4.8% of the Brazilian Amazon, which omits a large portion of rural and forested areas from their analysis (Figure 1). Because there is no information about the excluded health facilities or comparisons provided between included and excluded areas, it is highly likely that the analysis suffers from selection bias. This finding is perplexing, because the sophisticated modeling approach used can easily be extended to account for incomplete data. Given the wealth of research in rural and newly established areas that show a strong frontier malaria effect after deforestation, the exclusion of these types of areas should be strongly justified before reaching conclusions about any relationship between the environment and the disease.
Figure 1.
Valle and Clark1 study locations. This map shows the cities used in the study by Valle and Clark1 with 20-km buffers as used in their analysis. The yellow areas outline the extent of the Legal Brazilian Amazon and show the large area (> 95%) of the Brazilian Amazon that was not included in their analysis. Protected areas (PAs) referenced in the study by Valle and Clark1 and this article are shown in green.
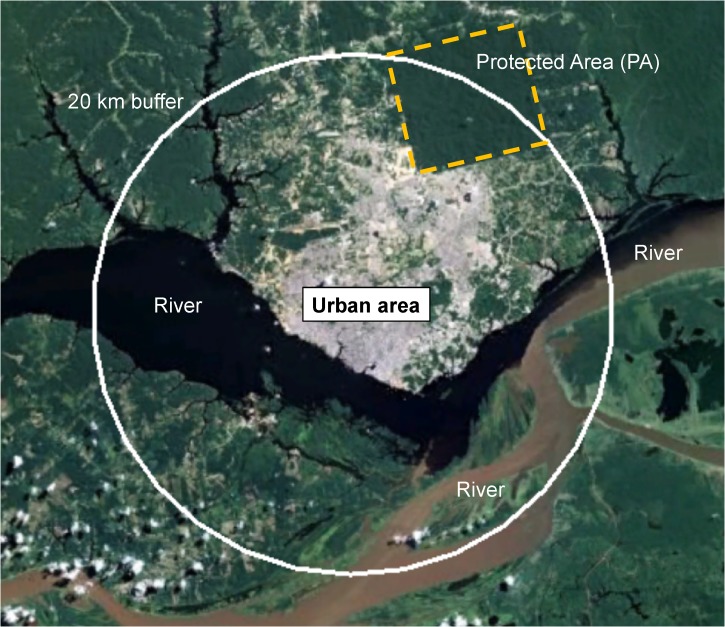
The 20-km buffer used by Valle and Clark1 (Figure 2) suffers from a classic ecological fallacy caused by aggregating exposure (proximity to a protected area [PA]) and disease (rural malaria cases), which results in false conclusions about the directionality of the relationship. Just 14.4% of malaria cases in Brazil occur in urban residents, whereas 24.6% originate in rural provisional settlements, indigenous areas, and mining outposts, which represent an enormous burden relative to the small population size in rural areas.14
Figure 2.
Map of Manaus with a 20-km buffer. This map uses Manaus, the largest city in the Amazon, as an example of one of the study areas in the work by Valle and Clark1 to show the potential issues with their analytical approach. The 20-km buffer that they used in their analysis covers almost the entire urban area (grey) and some of the rural area. The city has a large forest reserve (PA limits shown with a yellow dotted line), which is within the 20-km buffer. According to the analysis by Valle and Clark,1 they would likely find a high number of malaria cases associated with high forest cover because of the forest reserve. However, the malaria cases that would be reported to the health department in Manaus would not originate in the city or the forest reserve (which is not inhabited). The cases would occur in the rural populations along the rivers and creeks outside the 20-km buffer.
Although the Programa de Cálculo do Desflorestamento da Amazônia (PRODES) data used by Valle and Clark1 are the recognized standard for deforestation monitoring in the Amazon,15,16 the crude land cover classes (e.g., forest versus non-forest) are insufficient to draw the sweeping conclusion that “greater forest cover (as a proxy for proximity to forest fringes) tends to be associated with higher malaria incidence.” As discussed by Messina and Pan,17 this method is an unfortunate and often misused method that creates ecological fallacies because of the inability of coarse land classification products like PRODES to tease out subtle types of deforestation pressure when studying the impacts on malaria. For example, Vittor and others4,5 found that the presence of Anopheles darlingi larvae, A. darlingi biting rates, and human P. vivax rates (Amy Vittor, personal communication) were higher in areas near secondary regrowth forest but the opposite when they considered primary forest edge. Hahn MB and others18 did not detect the relationship between deforestation and malaria incidence at the county level, which has been found by others at the subcounty scale.3 However, Hahn MB and others (unpublished data) found that counties that had experienced selective logging saw a 72% increase in malaria risk compared with areas with no selective logging. Valle and Clark1 claim that they are measuring the impact of “conservation efforts,” but by failing to distinguish areas of secondary regrowth or selective logging from primary forests, they could just as easily be measuring the impact of logging from prior decades.1
Valle and Clark1 also ignore the fate of the cleared forest in their analysis.19 Deforestation in the Amazon has a number of drivers, including mining, cattle farming, soybean production, and urban sprawl,20 and the landscape created by each of these land uses has a different effect on malaria. For instance, in Mato Grosso, the southernmost state of the Amazonian region, forest is being replaced by cattle farming and large soybean plantations, and this use has reduced malaria incidence.14,21 However, 43% of cases in the Amazon are now being reported from Para, where deforestation results from timber production and mining.14
Another finding of Valle and Clark1 is that proximity to a PA is associated with increased malaria risk. According to the Brazilian National Protected Areas System (SNUC), there are two types of PAs: one type in which strict protection and biodiversity conservation are the primary objectives and another type defined as areas of sustainable use that allow varying levels of resource extraction.22 The effect of aggregating PAs into one group is akin to using crude land cover classes resulting in aggregation bias. A prime example is in Acre, where rubber-tapping communities have PAs designated for sustainable use such that forest cover remains constant, but human–environment relationships are also persistent.22 In this setting, rubber tapping has been identified as a key determinant of malaria risk.9 Related to this finding is recent research by Nolte and others23 that showed both the importance of PA type on human–environment interactions as well as the tendency of PAs to be located in areas of high deforestation pressure. Because cities included in the analysis were likely larger with more established infrastructure (cities with missing data were excluded), one can also assume that these cities exert higher pressure on the surrounding landscape than excluded cities and thus, are more likely to be located near a PA. It is important to note that, of 36 sustainable use PAs, 28 PAs are in the Amazon, and of 441 indigenous PA reserves, 361 PAs are in the Amazon (20% of its total area).22,24 Although Valle and Clark1 raise a valid point that potential negative impacts of PAs have not been vetted, to properly evaluate the impact of proximity to PAs, one would need to at least adjust for the potential location bias of a PA and its type and include towns of all sizes.
We conclude that a thorough understanding of the complex relationship between ecological disturbance and malaria across the Brazilian Amazon requires continuous collection of malaria data at the submunicipality level, high-resolution satellite imagery that can detect subcanopy forest disturbances (e.g., selective logging), and accurate sociodemographic data to address potentially large confounders, such as migration, access to healthcare, and occupational exposure. We applaud the Brazilian Ministry of Health for publishing comprehensive data of confirmed malaria cases, which supports regional geospatial analyses, but there are limitations.25 The connections between forest disturbance and malaria in the Brazilian Amazon have been described within many spatial, temporal, and social contexts, but the complexity of the disease transmission cycle and the paucity of environmental and health data at similar spatial and temporal resolutions have made broad conclusions difficult to establish. Regardless, the edge of the Brazilian Amazon is among the most active land use frontiers26 and the largest remaining tropical forest.26 Policymakers need strong science-based guidance to make land use decisions that will inevitably affect global and regional climate, biodiversity, and human health.
ACKNOWLEDGMENTS
We thank Steve Osofsky, Lynne Gaffikin, and the Health & Ecosystems: Analysis of Linkages (HEAL) program for their assistance in identifying recent studies. We also thank George Allez for editing the manuscript.
Footnotes
Authors' addresses: Micah B. Hahn and Jonathan A. Patz, Nelson Institute, Center for Sustainability and the Global Environment (SAGE), Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin, Madison, WI, E-mails: mbhahn@wisc.edu and patz@wisc.edu. Sarah H. Olson, Wildlife Conservation Society—Canada, Nanaimo, British Columbia, Canada, E-mail: solson@wcs.org. Amy Y. Vittor, Division of Infectious Diseases, Department of Medicine, University of Pennsylvania, Philadelphia, PA, and Department of Pathology, University of Texas, Galveston, TX, E-mail: amy.vittor@uphs.upenn.edu. Christovam Barcellos, Health Information Research Department, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil, E-mail: xris@fiocruz.br. William Pan, Nicholas School of the Environment, Duke University, Durham, NC, E-mail: william.pan@duke.edu.
References
- 1.Valle D, Clark J. Conservation efforts may increase malaria burden in the Brazilian Amazon. PLoS One. 2013;8:e57519. doi: 10.1371/journal.pone.0057519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laporta GZ, De Prado PIKL, Kraenkel RA, Coutinho RM, Sallum MAM. Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl Trop Dis. 2013;7:e2139. doi: 10.1371/journal.pntd.0002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mâncio Lima County, Brazil. Emerg Infect Dis. 2010;6:1108–1115. doi: 10.3201/eid1607.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Sánchez-Lozano W, Pinedo VV, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 5.Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sánchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, Patz JA. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12. [PMC free article] [PubMed] [Google Scholar]
- 6.Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000;6:103–1089. doi: 10.3201/eid0602.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGreevy PB, Dietze R, Prata A, Hembree SC. Effects of immigration on the prevalence of malaria in rural areas of the Amazon basin of Brazil. Mem Inst Oswaldo Cruz. 1989;84:485–491. doi: 10.1590/s0074-02761989000400005. [DOI] [PubMed] [Google Scholar]
- 8.Singer BH, De Castro MC. Agricultural colonization and malaria on the Amazon frontier. Ann N Y Acad Sci. 2001;954:184–222. doi: 10.1111/j.1749-6632.2001.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 9.Caldas de Castro MC, Monte-Mór RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci USA. 2006;103:2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caviglia-Harris JL, Sills EO, Mullan K. Migration and mobility on the Amazon frontier. Popul Environ. 2012;34:338–369. [Google Scholar]
- 11.Vasconcelos CH, Novo ELMM, Donalisio MR. Use of remote sensing to study the influence of environmental changes on malaria distribution in the Brazilian Amazon. J Public Health (Bangkok) 2006;22:517–526. doi: 10.1590/s0102-311x2006000300006. [DOI] [PubMed] [Google Scholar]
- 12.Griffing SM, Viana GMR, Mixson-Hayden T, Sridaran S, Alam MT, De Oliveira AM, Barnwell JW, Escalante A, Povoa MM, Udhayakumar V. Historical shifts in Brazilian P. falciparum population structure and drug resistance alleles. PLoS One. 2013;8:e58984. doi: 10.1371/journal.pone.0058984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística (IBGE) Brazilian Census. 2010. http://www.ibge.gov.br/english/ Available at. Accessed May 16, 2013. [Google Scholar]
- 14.HSS-BMoH . Situação epidemiológica da malária no Brasil, 2000 a 2011. Boletim Epidemiológico. 2013. p. 44.http://portalsaude.saude.gov.br/portalsaude/index.cfm?portal=pagina.visualizarTexto&codConteudo=10252&codModuloArea=783&chamada=boletim-epidemiologico-da-malaria-_-2013 Available at. Accessed May 24, 2013. [Google Scholar]
- 15.Asner GP, Broadbent EN, Oliveira PJC, Keller M, Knapp DE, Silva JNM. Condition and fate of logged forests in the Brazilian Amazon. Proc Natl Acad Sci USA. 2006;103:12947–12950. doi: 10.1073/pnas.0604093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Câmara G, De Morrisson Vaeriano D, Soares J. Methodology for the Calculation of Annual Deforestation Rates in the Amazon. São Paulo, Brazil: National Institute for Space Research; 2006. [Google Scholar]
- 17.Messina JP, Pan W. Different ontologies: land change science and health research. Curr Opin Environ Sustain. 2013;5:515–521. doi: 10.1016/j.cosust.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn MB, Gangnon RE, Barcellos C, Asner GP, Patz J. Influence of deforestation, logging, and fire on malaria in the Brazilian Amazon. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085725. e85725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton DC, DeFries RS, Shimabukuro YE, Anderson LO, Arai E, Del Bon Espirito-Santo F, Freitas R, Morisette J. Cropland expansion changes deforestation dynamics in the southern Brazilian Amazon. Proc Natl Acad Sci USA. 2006;103:14637–14641. doi: 10.1073/pnas.0606377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambin EF, Geist HJ, Lepers E. Dynamics of land use and land cover change in tropical regions. Annu Rev Environ Resour. 2003;28:205–241. [Google Scholar]
- 21.Atanaka-Santos M, Souza-Santos R, Czeresnia D. Spatial analysis for stratification of priority malaria control areas, Mato Grosso State, Brazil. Cad Saude Publica. 2007;23:1099–1112. doi: 10.1590/s0102-311x2007000500012. [DOI] [PubMed] [Google Scholar]
- 22.Rylands AB, Brandon K. Brazilian protected areas. Conserv Biol. 2005;19:612–618. [Google Scholar]
- 23.Nolte C, Agrawal A, Silvius KM, Soares-Filho BS. Governance regime and location influence avoided deforestation success of protected areas in the Brazilian Amazon. Proc Natl Acad Sci USA. 2013;110:4956–4961. doi: 10.1073/pnas.1214786110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartzman S, Zimmerman B. Conservation alliances with indigenous peoples of the Amazon. Conserv Biol. 2005;19:721–727. [Google Scholar]
- 25.Ministerio da Saude . SIVEP-Malaria. 2010. http://portalweb04.saude.gov.br/sivep_malaria/ Available at. Accessed May 29, 2013. [Google Scholar]
- 26.Food and Agriculture Organization . Global Forest Resources Assessment 2005: Progress Towards Sustainable Forest Management. Rome, Italy: United Nations; 2006. [Google Scholar]
- 27.Foley JA, Asner GP, Costa MH, Coe MT, DeFries R, Gibbs HK, Howard EA, Olson S, Patz J, Ramankutty N, Snyder P. Amazonia revealed: forest degradation and loss of ecosystem goods and services in the Amazon Basin. Front Ecol Environ. 2007;5:25–32. [Google Scholar]