Figure 3. TGF-β expression corresponds to synaptic refinement period in the retinogeniculate system.
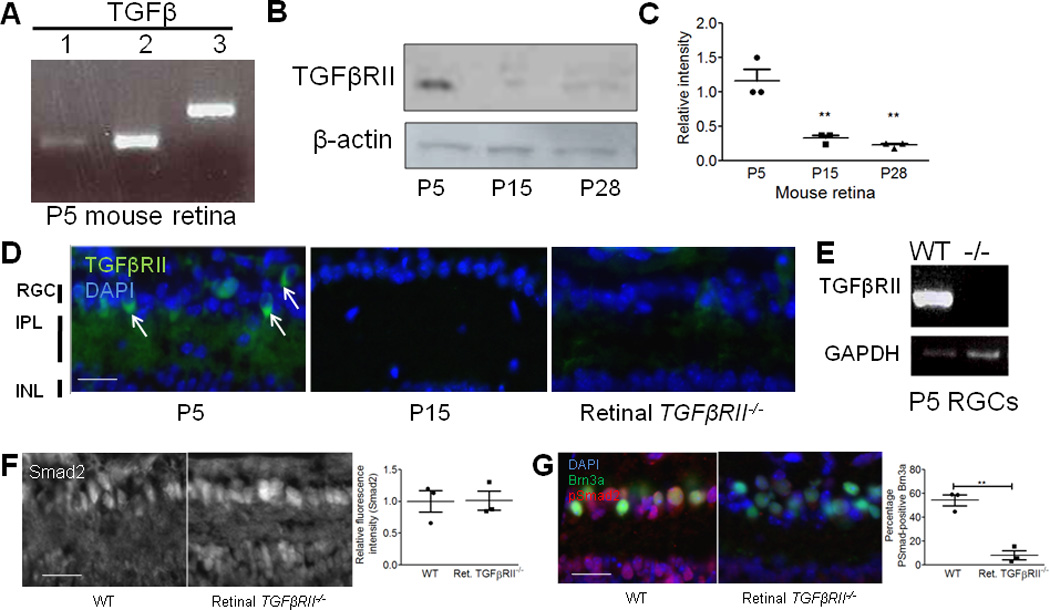
(A) RT-PCR showed expression of all three TGF-β isoforms in the P5 mouse retina. Data are representative of 4 mice. (B) Western blot for TGFβRII (goat anti-human TGFβRII, R&D systems) showed developmental expression of TGFβRII in the mouse retina. See Supplemental Fig. 6 for full length blot. (C) Relative intensity quantification normalized to beta-actin control for each age showed developmental TGFβRII expression in the postnatal mouse retina (one-way ANOVA, n=3 experiments, **p<0.01, F(2,6)=26.36). (D) Immunostaining with antibodies against TGFβRII (R&D systems, goat anti-TGFβRII) showed that the receptor localizes to the RGC layer and the IPL (arrows) and that staining intensity is dramatically reduced at P15 relative to P5. Antibody staining was confirmed for specificity by staining retinal TGFβRII−/− mice. All images were obtained with set exposure times. Scale bar = 50um. (E) RT-PCR confirmed the absence of tgfbr2 mRNA in RGCs acutely isolated from P5 mice using immunopanning. Data shown are representative of 4 animals tested. Full length gel in Supplemental Fig. 6. (F) Immunohistochemistry for total Smad2 showed no difference in relative fluorescence intensity (RGC layer) in WT littermates and retinal TGFβRII−/− mice (t test, n=3 mice/genotype, p=0.96 (ns), t(4)=0.053). Scale bar = 50µm. (G) Immunohistochemistry for phosphorylated Smad (pSmad). Co-staining for an RGC marker, Brn3a, and pSmad2/3 showed a significant reduction in pSmad levels quantified in RGCs specifically (t test, n= 3 mice/group, p<0.001, t(4)=13.18). Scale bar = 50µm.
