Table 2. Scope of O-nitroso aldol reaction.
| Entry | 4 | Yield, %* | 5/7† | ee of 5, %‡ | (Conf.)§ | |
|---|---|---|---|---|---|---|
| 1 |
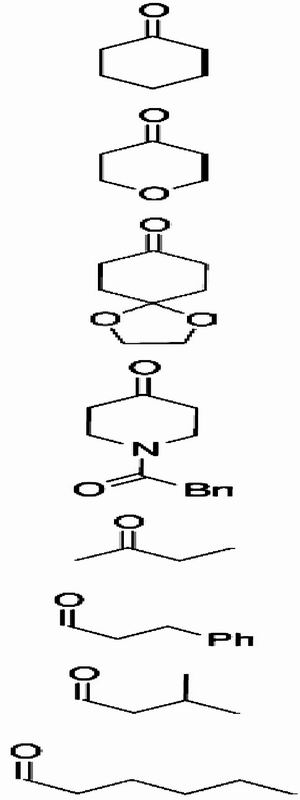
|
4a | 94 | >99/- | >99 | (R) |
| 2 | 4b | 87 | >99/- | >99 | ||
| 3 | 4c | 97 | >99/- | 99 | ||
| 4 | 4d | 95 | >99/- | >99 | ||
| 5¶ | 4e | 75 | 72/28 | >99 | ||
| 6** | 4f | 67∥ | >99/- | 98 | (R) | |
| 7†† | 4g | 65∥ | >99/- | 98 | ||
| 8** | 4h | 69∥ | >99/- | 98 |
Reactions were conducted with 5 mol % of 3f, 1.0 eq of nitrosobenzene, and 3 eq of 4 in DMSO at rt.
Isolated yield.
Determined by yield of each isolated isomer.
Determined by HPLC (Supporting Text).
Determined after conversion to the corresponding diol (Supporting Text).
Reaction was conducted with 20 mol % of 3f in DMSO at rt.
Determined by isolated yield of corresponding primary alcohol obtained after reduction of product.
Reactions were conducted with 10 mol % of 3f in MeCN at rt.
Reaction was conducted with 20 mol % of 3f in MeCN at rt.
