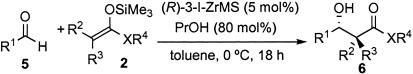
Table 8.
| Entry | 1; R1 | Si enolate | Yield, % | syn/anti | ee, % (anti) |
|---|---|---|---|---|---|
| 1 | Ph (5a) | 2a | 92 | — | 94 |
| 2 | Ph (5a) | 2b | Quant. | — | 92 |
| 3* | Ph (5a) | 2b | 97 | — | 94 |
| 4 | Ph (5a) | 2f | Quant. | 5/95 | 99 |
| 5 | p-MeOC6H4 (5b) | 2f | 80 | 5/95 | 94 |
| 6 | p-ClC6H4 (5c) | 2f | Quant. | 8/92 | 95 |
| 7 | PhCH=CH (5d) | 2f | 94 | 16/84 | 98 |
| 8 | PhCH2CH2 (5e) | 2f | 65 | 15/85 | 87 |
Quant., quantitative.
Catalyst (10 mol %) was used.