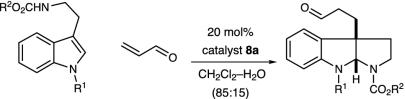
Table 2.
Enantioselective pyrroloindoline formation with representative N(1)- and N(10)-substituted tryptamines
| Entry | R1 | R2 | Time, h | Yield, % | ee,* % |
|---|---|---|---|---|---|
| 1 | Allyl | t-Bu | 25 | 85 | 89 |
| 2 | Allyl | Et | 26 | 89 | 89 |
| 3 | Prenyl | Et | 24 | 89 | 89 |
| 4 | Benzyl | Allyl | 48 | 83 | 89 |
| 5 | Benzyl | t-Bu | 30 | 82 | 90† |
Product ratios determined by HPLC.
Absolute configuration determined by chemical correlation.